Press release
Reprocessed Single-Use Devices Market to Grow at 13.8% CAGR Through 2032, Persistence Market Research Reports
IntroductionIn the evolving landscape of healthcare, cost pressures, sustainability concerns, and regulatory changes are driving novel strategies. One of the standout trends is the reprocessing of single-use medical devices (SUDs). Instead of discarding devices after one use, certain classes of single-use devices can be cleaned, sterilized, validated, and reused under stringent controls. This practice offers potential savings, waste reduction, and resource efficiency-if done safely and in compliance with regulations.
Download Sample PDF Of This Report:
https://www.persistencemarketresearch.com/samples/13870
According to a leading forecast, the global reprocessed single-use devices market size is likely to value at US$ 1.9 Bn in 2025 and reach US$ 4.7 Bn by 2032, growing at a CAGR of 13.8% during the forecast period from 2025 to 2032. This robust growth projection underlines both the opportunity and the challenges ahead for stakeholders across healthcare, manufacturing, and regulation.
In this article, we explore the market dynamics, growth drivers, segmentation, regional outlook, challenges, and competitive landscape for the reprocessed single-use devices market through 2032.
Market Outlook & Forecast (2025-2032)
• Base & Forecast Values
In 2025, the market is forecast at USD 1.9 billion, serving as the base for the projection window. By 2032, it is expected to swell to USD 4.7 billion, reflecting more than doubling in size over seven years at a compounded annual growth rate (CAGR) of 13.8%.
• Growth Trajectory
A CAGR of 13.8% implies a healthy and sustainable expansion, balanced between early adoption and maturity. The market will likely see accelerating growth in the early years (2025-2028) as reprocessing protocols, validation, and regulatory acceptance mature. Growth may moderate slightly toward the end of the period as saturation in advanced markets sets in.
• Market Share & Positioning
With this forecast, reprocessed single-use devices will become an increasingly prominent niche in the broader medical devices and medical reprocessing markets. They will likely carve out share from both new single-use purchases and wholly reusable device options.
For More Details: https://www.persistencemarketresearch.com/market-research/reprocessed-single-use-devices-market.asp
Key Drivers Fueling Growth
Several interlinked drivers underpin the promising growth outlook:
1. Cost Containment Pressures in Healthcare
Hospitals, surgical centers, and health systems globally are under pressure to reduce operating costs. Reprocessing single-use devices offers potential savings-sometimes 20-50% or more compared to buying new equivalents-while retaining comparable performance (if validated).
2. Waste Reduction & Sustainability Imperatives
Medical waste is a major environmental and regulatory concern. Reprocessing helps reduce the volume of discarded devices, packaging, and associated waste streams. Many health systems are committing to "green hospital" or circular economy goals, making reprocessing more attractive.
3. Advances in Sterilization, Cleaning & Validation Technologies
Continued improvements in sterilants (e.g., low-temperature gas plasma, hydrogen peroxide vapor), cleaning automation, traceability, and validation methods are enhancing safety, consistency, and confidence in reprocessed products.
4. Increasing Procedure Volumes and Medical Device Utilization
As surgical and interventional procedures grow globally (driven by population aging, chronic disease burden, and improving access), the demand base for single-use devices expands-offering more opportunities for reprocessing.
5. Regulatory Support & Clarity
In markets like the U.S., reprocessors must comply with stringent regulatory oversight (e.g., FDA rules) to ensure that reprocessed devices are as safe and effective as originals. Over time, clearer guidance, standards, and case precedent help reduce adoption risk. (See FDA's guidance on reprocessing single-use devices.)
6. Healthcare Infrastructure Upgrade in Emerging Markets
In many emerging economies, acquisition budgets are limited, making cost-effective solutions essential. As hospital and surgical capacity expand, reprocessing offers a way to contain per-case device costs.
7. Third-Party Reprocessors & Outsourcing Models
Many healthcare providers prefer to outsource reprocessing to specialized players (third-party reprocessors) rather than invest heavily in in-house infrastructure. This enables scale, specialization, and compliance, and accelerates adoption.
Segmentation Analysis
Understanding how the market splits helps identify high-opportunity sub-segments.
By Device Class / Category
• Class I / Low-risk Devices: Devices such as tourniquet cuffs, pulse oximeter sensors, and other devices categorized as low risk. These are easier to revalidate, and often serve as entry points for reprocessing adoption.
• Class II / Higher-risk Devices: Catheters, guidewires, and interventional devices. These require more stringent validation, but tend to deliver greater cost benefits per unit.
Typically, the Class II segment commands a higher share due to higher prices and reuse gain margin.
By Procedure / Application
• Cardiology / Vascular Devices: Catheters and wires are heavily used in interventional cardiology; hence, they often dominate in many reprocessing forecasts.
• General Surgery / Laparoscopic Tools / Scalpels / Forceps: These are common across many hospitals, making them viable for reprocessing if validated.
• Diagnostic & Endoscopic Devices: Endoscopes or single-use scopes potentially form a niche but require advanced cleaning regimes.
• Orthopedic / Other Specialties: Some reusable or semi-reusable surgical kits or instruments may also be included, although they present tougher validation challenges.
By End User
• Hospitals & Health Systems: As the largest volume users of disposable devices, hospitals are prime candidates for reprocessing adoption.
• Ambulatory Surgery Centers (ASCs): These smaller, procedure-focused centers may adopt reprocessing to reduce per-case costs.
• Specialty Clinics / Outpatient Facilities: Some reprocessed devices may find use in smaller settings, depending on regulatory allowances.
• Others (e.g., Home Care, Field Clinics): In niche uses, reprocessed devices could play a role, particularly in cost-constrained settings.
By Operational Model
• In-house Reprocessing: Hospitals maintain their own reprocessing units. While offering control, this demands capital, regulatory compliance, and staffing.
• Third-party / Outsourced Reprocessing: More popular in many markets, this model outsources cleaning, sterilization, validation, and return logistics to certified reprocessors.
Regional Outlook & Market Share
The market will not grow uniformly across geographies; regional dynamics matter.
North America
North America is expected to remain a dominant market due to established regulations, strong healthcare infrastructure, high device usage, and earlier adoption of reprocessing protocols. U.S. regulatory clarity under agencies like the FDA supports trust in reprocessed devices.
Europe
Some European nations permit or encourage reprocessing; however, adoption is uneven due to regulatory variability, reimbursement rules, and procurement practices. Over time, regulatory harmonization and environmental mandates may push growth.
Asia Pacific
Rapid growth is expected in the Asia Pacific region owing to expanding healthcare access, rising surgical volumes, constrained budgets, and increasing environmental awareness. Nations like India, China, Southeast Asia could be strong markets if regulatory frameworks evolve.
Latin America & Middle East & Africa
These regions are nascent in adoption but represent opportunity zones. Cost constraints may drive interest, but regulatory and infrastructure hurdles (sterilization logistics, traceability, quality control) can slow adoption.
Challenges & Risks
While the outlook is favorable, several barriers remain:
• Regulatory & Liability Concerns: Ensuring that a reprocessed device is as safe and effective as a new one is nontrivial. Liability, approvals, and post-market surveillance are critical.
• Clinical Resistance / Trust Issues: Some clinicians may resist use of reprocessed devices due to perceived risk, reputation, or lack of familiarity.
• Validation & Quality Control Costs: Rigorous testing, traceability (barcoding, lot control), and sterilization must meet high standards, which demand investment.
• Material Degradation & Device Design Limitations: Not all single-use devices are amenable to reprocessing; materials may degrade, residual residues may remain, or device geometry may limit cleaning.
• Logistical Complexities: Collection, transport, decontamination, sterilization, inspection, redistribution-each step adds complexity and cost.
• Market Fragmentation & Scale Effects: Smaller hospitals may not have sufficient volume to justify in-house reprocessing infrastructure; lack of scale can erode margins.
Competitive Landscape & Key Players
Several types of players compete or participate:
• Specialist Reprocessing Providers: Companies dedicated to reprocessing single-use devices, with expertise in sterilization, validation, and logistics.
• Large Medical Device / OEM Firms: Some original equipment manufacturers may incorporate reprocessing strategies or partner with reprocessors.
• Hospitals / Health Systems with In-house Programs: Large hospital networks may internalize reprocessing, especially for high-use items.
• Regulatory & Standards Bodies: Organizations issuing guidelines, auditing compliance, and influencing adoption.
Prominent names in the space may include (but are not limited to) Stryker Sustainability Solutions, Medline (renewal divisions), SterilMed, ReNu Medical, and others-though local and regional players also play an important role.
Outlook & Strategic Considerations for Stakeholders
• For Healthcare Providers: Evaluate devices with high reuse potential (e.g., catheters, wires, laparoscopic tools) and conduct pilot reprocessing programs. Negotiate contracts with certified reprocessors.
• For Reprocessor Firms: Invest in robust validation, traceability (barcodes, RFID), sterilization technologies, and regulatory compliance capabilities to gain trust and scale.
• For Regulators & Policymakers: Provide clear, consistent guidance on reprocessing, inspections, liability, and reimbursement incentives aligned with sustainability goals.
• For Device OEMs: Consider designing devices with reprocessing in mind (i.e., materials, surfaces, modularity) or offering "remanufacturable versions" in collaboration with reprocessing firms.
As market adoption increases, consolidation, partnerships, and vertical integration (OEM + reprocess) may reshape the competitive landscape.
Summary & Outlook to 2032
The reprocessed single-use devices market is poised for strong expansion from USD 1.9 billion in 2025 to USD 4.7 billion in 2032, at a projected CAGR of 13.8%. The growth is underpinned by pressures on healthcare costs, sustainability mandates, advances in cleaning/sterilization technologies, and maturing regulatory ecosystems.
Nevertheless, adoption will vary across geographies and device types. The early growth will center around mid-risk devices and settings with sufficient volume to justify reprocessing. Over time, as trust, validation protocols, and logistics mature, more device classes and more markets will join.
If healthcare systems, regulators, and industry players coordinate well-balancing innovation, safety, cost, and environmental gains-the reprocessed single-use devices market may become an integral pillar of efficient, sustainable care delivery.
Explore the Latest Trending Research Reports:
Wound Cleanser Products Market- https://www.persistencemarketresearch.com/market-research/wound-cleanser-products-market.asp
Atomic Magnetometers Market- https://www.persistencemarketresearch.com/market-research/atomic-magnetometers-market.asp
`
About Persistence Market Research:
At Persistence Market Research, we specialize in creating research studies that serve as strategic tools for driving business growth. Established as a proprietary firm in 2012, we have evolved into a registered company in England and Wales in 2023 under the name Persistence Research & Consultancy Services Ltd. With a solid foundation, we have completed over 3600 custom and syndicate market research projects, and delivered more than 2700 projects for other leading market research companies' clients.
Our approach combines traditional market research methods with modern tools to offer comprehensive research solutions. With a decade of experience, we pride ourselves on deriving actionable insights from data to help businesses stay ahead of the competition. Our client base spans multinational corporations, leading consulting firms, investment funds, and government departments. A significant portion of our sales comes from repeat clients, a testament to the value and trust we've built over the years.
Contact Us:
Persistence Market Research
G04 Golden Mile House, Clayponds Lane
Brentford, London, TW8 0GU UK
USA Phone: +1 646-878-6329
UK Phone: +44 203-837-5656
Email: sales@persistencemarketresearch.com
Web: https://www.persistencemarketresearch.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Reprocessed Single-Use Devices Market to Grow at 13.8% CAGR Through 2032, Persistence Market Research Reports here
News-ID: 4219998 • Views: …
More Releases from Persistence Market Research

Cryogenic Storage Tanks Market Predicted to Hit US$ 12.8 Billion by 2033 Driven …
According to the latest study by Persistence Market Research, the global cryogenic storage tanks market is likely to be valued at US$ 8.6 billion in 2026 and is projected to reach US$ 12.8 billion by 2033, expanding at a CAGR of 5.8% during the forecast period 2026-2033. Rising demand for liquefied gases across energy, healthcare, food processing, and industrial manufacturing sectors is emerging as a key driver shaping the market's…
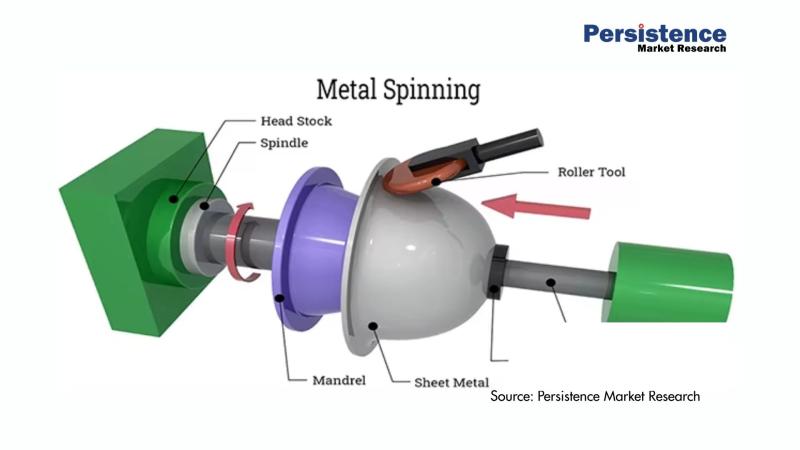
Metal Spinning Products Market Projected to Grow to US$ 4.0 billion by 2033 - Pe …
The global metal spinning products market is poised for substantial growth in the coming years. According to a recent study by Persistence Market Research, the market size is anticipated to reach US$ 4.0 billion by 2033, growing at a robust compound annual growth rate (CAGR) of 4.2% from its current valuation of US$ 3.0 billion in 2026. Metal spinning, a process of shaping metal into precise and symmetrical shapes, is…
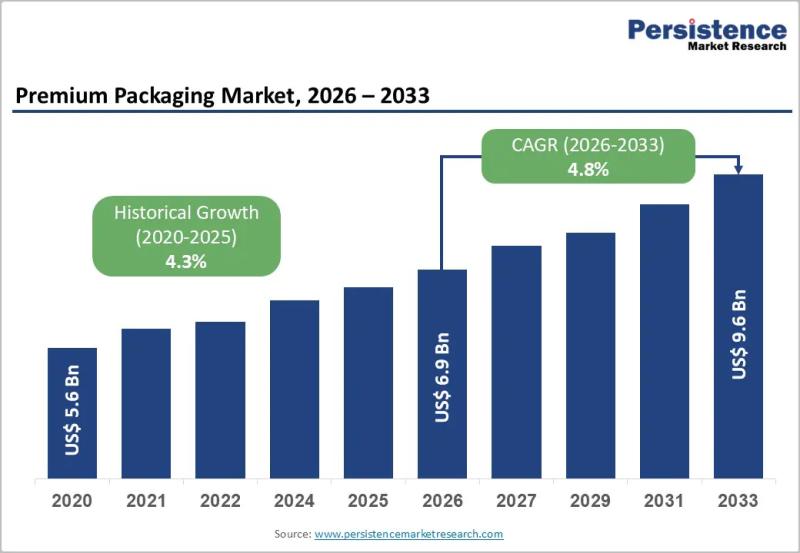
Premium Packaging Market Size Worth US$9.6 Billion by 2033 - Persistence Market …
The premium packaging market has evolved into a critical strategic element for brand differentiation across multiple high value consumer industries. Premium packaging goes beyond basic containment and protection to deliver enhanced aesthetics tactile appeal storytelling and emotional connection. Brands increasingly view packaging as an extension of their identity and a powerful marketing tool that influences purchasing decisions at the point of sale and during the unboxing experience. This shift is…
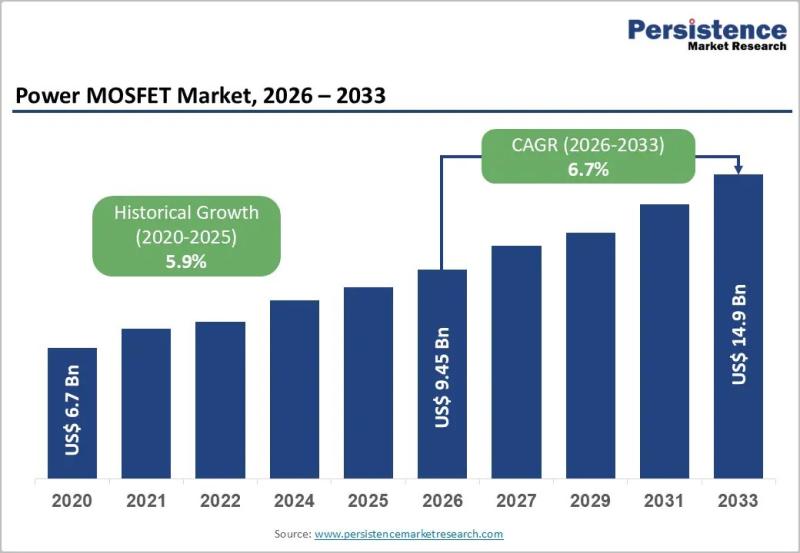
Power MOSFET Market Growth Driven by EVs Renewable Energy and Smart Automation
The global Power MOSFET market is entering a phase of sustained expansion, driven by the accelerating need for energy-efficient and high-performance power management components across industries. In 2026, the market is expected to be valued at US$ 9.45 billion and is forecast to reach US$ 14.9 billion by 2033, registering a healthy CAGR of 6.7% during the forecast period. Power MOSFETs are essential semiconductor devices that enable efficient switching and…
More Releases for Device
Medical Device Regulatory Affairs Market Medical Device Regulatory Affairs Marke …
"Medical Device Regulatory Affairs Market" in terms of revenue was estimated to be worth $ 6.7 billion in 2024 and is poised to reach $ 18.3 billion by 2034, growing at a CAGR of 10.8% from 2025 to 2034 according to a new report by InsightAce Analytic.
Request For Free Sample Pages:
https://www.insightaceanalytic.com/request-sample/1913
Latest Drivers Restraint and Opportunities Market Snapshot:
Key factors influencing the global medical device regulatory…
Surge In Wireless Device Usage Boosts Wireless Audio Device Market Driving Marke …
Stay ahead with our updated market reports featuring the latest on tariffs, trade flows, and supply chain transformations.
How Large Will the Wireless Audio Device Market Size By 2025?
In recent years, there has been remarkable growth in the wireless audio device market size. The market, which is projected to expand from $41.85 billion in 2024 to $52.37 billion in 2025, boasts a compound annual growth rate (CAGR) of 25.1%. Factors contributing…
Anti-snoring Device Market - Quiet Nights, Restful Sleep: Anti-snoring Device In …
Newark, New Castle, USA: The "Anti-snoring Device Market" provides a value chain analysis of revenue for the anticipated period from 2023 to 2031. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors.
Anti-snoring Device Market: https://www.growthplusreports.com/report/antisnoring-device-market/8931
This latest report researches the industry structure, sales, revenue,…
Global Watch Clock Measuring Device Market | Watch Clock Measuring Device Indust …
Watch, clock and measuring device market comprises of the sales of watch, clock, measuring device & related services to measure the time and physical quantity. Watch is portable timepiece, which is worn by people around the wrist, attached by a strap. Clock is a device used to measure and indicate time, using the pointers moving over a dial. Measuring device is an instrument used for measuring the various parameters in…
Peripheral Vascular Device Market Size, Peripheral Vascular Device Market Share, …
Global Peripheral Vascular Device Market Size is observed to gain traction owing to the factors such as increasing research and development for developing several new product, and rising funding by the private organizations.
Request for Sample of This Research Report @ https://bit.ly/2xjOKpC
Top Key Player:-
Abbott Laboratories
Braun Melsungen AG
Boston Scientific Corporation
R. Brad, Inc.
Cardinal Health, Inc.
Medtronic plc.
Cook Medical, Inc.
Teruma Corporation
Jude Medical, Inc.
The Spectranetics Corporation
Volcano Corporation
Peripheral vascular disorder (PVD) is a blood circulation disorder…
Medical Device Technologies Market - The Evolution of Medical Device Technologie …
The global medical device technologies market is anticipated to be boosted by various well-known players in the market. Some of these players that are dealing with the manufacturing of in vitro diagnostic devices hold a significant share in the global market. Whereas, the small market players are emerging from several developing nations, looking to set their foot in the market. Such measures are foreseen to change the market scenario in…
