Press release
Parkinson Disease Pipeline Drugs 2025 Report: Emerging Drugs, Innovative Therapies, Clinical Trial Updates, and Top Companies
DelveInsight's, "Parkinson's Disease Pipeline Insight 2025" report provides comprehensive insights about 130+ companies and 150+ pipeline drugs in the Parkinson's Disease pipeline landscape. It covers the Parkinson's Disease pipeline drug profiles, including clinical and nonclinical stage products. It also covers the Parkinson's Disease pipeline therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.Discover the latest drugs and treatment options in the Parkinson's Disease Pipeline. Dive into DelveInsight's comprehensive report today! @ Parkinson's Disease Pipeline Outlook [https://www.delveinsight.com/sample-request/parkinsons-disease-pipeline-insights?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Key Takeaways from the Parkinson's Disease Pipeline Report
* On October 7, 2025 , a study published online in Neurology highlighted a potential link between ambient trichloroethylene (TCE) exposure and an increased risk of Parkinson's disease (PD). Researchers, led by Brittany Krzyzanowski, Ph.D., at the Barrow Neurological Institute in Phoenix, conducted a population-based, case-control study evaluating U.S. Medicare beneficiaries aged 67 and older between 2016 and 2018. The study assessed participants' residential exposure to ambient TCE in 2002 and estimated the relative risk of incident PD while adjusting for factors such as age, gender, race, smoking status, health care utilization, rural-urban commuting area, and fine particulate matter. The findings suggest that long-term environmental exposure to TCE may contribute to the development of PD in older adults.
* DelveInsight's Parkinson's Disease Pipeline report depicts a robust space with 130+ active players working to develop 150+ pipeline therapies for Parkinson's Disease treatment.
* The leading Parkinson's Disease Companies such as Annovis Bio, Cerevel Therapeutics, Roche, Inhibikase Therapeutics, Inc, Gain Therapeutics, Neuronascent, ANEW MEDICAL, Longevity Biotech, Minerva Neurosciences/Mitsubishi Tanabe Pharma Corporation, Tetraneuron, Kallyope, BlueRock Therapeutics LP, Neuraly, Peptron/Yuhan Corporation, Genentech, Kissei Pharmaceutical, Asklepios BioPharmaceutical, Alkahest, Modag, Intra-Cellular Therapies, Living Cell Technologies, MedImmune and others.
* Promising Parkinson's Disease Pipeline Therapies such as VTX3232, Rasagiline, Safinamide Methanesulfonate, Levetiracetam, CTC-413, CVXL-0107, Levodopa, Rotigotine, Istradefylline 20 mg or 40 mg, IPX054 100 mg and others.
Stay ahead with the most recent pipeline outlook for Parkinson's Disease. Get insights into clinical trials, emerging therapies, and leading companies with Parkinson's Disease @ Parkinson's Disease Treatment Drugs [https://www.delveinsight.com/sample-request/parkinsons-disease-pipeline-insights?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
The Parkinson disease Pipeline Report provides disease overview, pipeline scenario and therapeutic assessment of the key pipeline therapies in this domain. The Parkinson disease Pipeline Report also highlights the unmet needs with respect to the Parkinson disease.
Parkinson disease Overview
Parkinson's disease (PD) is a chronic and progressive neurological disorder that primarily affects movement. It is characterized by the gradual loss of dopamine-producing neurons in the substantia nigra, a region of the brain crucial for movement control. PD typically manifests in individuals over the age of 60, though younger onset is possible. It is the second most common neurodegenerative disorder after Alzheimer's disease. The progression of symptoms can vary widely among individuals, making the disease challenging to predict and manage.
Parkinson's Disease Emerging Drugs Profile
* Buntanetap: Annovis Bio
Buntanetap (formerly known as Posiphen or ANVS401) targets neurodegeneration by inhibiting the formation of multiple neurotoxic proteins, including amyloid beta, tau, alpha-synuclein, and TDP43. By improving synaptic transmission, axonal transport, and reducing neuroinflammation, Buntanetap aims to reverse neurodegeneration in AD, PD, and other neurodegenerative diseases, thereby aiming to restore brain function and improve the quality of life for patients. Currently, the drug is in Phase III trial for the treatment of Parkinson's disease.
* Tavapadon: Cerevel Therapeutics
Tavapadon is the first and only selective D1/D5 receptor partial agonist in development for Parkinson's disease and is currently being studied as a once-daily medicine for use as both a monotherapy and as an adjunctive therapy to LD. Tavapadon is designed to selectively and optimally activate D1/D5 receptors to potentially provide the right balance of motor control, safety and tolerability for patients. By selectively activating D1/D5 dopamine receptors along the nigrostriatal pathway, tavapadon has the potential to offer the right balance of dopamine signaling to improve motor control while avoiding D2/D3 overstimulation, which is believed to underlie many of the side effects of current dopamine agonists. Additionally, as a partial agonist with a 24-hour half-life enabling once-daily dosing, tavapadon may avoid hyper activation of the dopamine receptors, which can lead to troublesome dyskinesias. Currently, the drug is in Phase III trial for the treatment of Parkinson's disease.
* Prasinezumab: Roche
Prasinezumab (RG7935) is a monoclonal antibody targeting alpha-synuclein, a protein that may misfold and be involved in the pathogenesis of Parkinson's disease. It has been tested in preclinical models of synuclein-related disease and has shown a reduction of neurodegeneration. Currently, the drug is in Phase II trial for the treatment of Parkinson's disease.
* Risvodetinib: Inhibikase Therapeutics, Inc
Risvodetinib (IkT-148009), is a potent, selective small-molecule designed and engineered as chronically administered, once-daily oral medication targeting the underlying biological mechanism resulting in Parkinson's disease, with the goal of halting disease progression and reversing functional loss. Risvodetinib (IkT-148009) is designed to block the activation of Abl kinase, a clinically validated drug target, to halt and reverse the loss of dopamine-secreting neurons in the brain and GI tract by restoring neuroprotective mechanisms. Currently, the drug is in Phase II trial for the treatment of Parkinson's disease.
* GT 02287: Gain Therapeutics
GT-02287, is an allosteric regulator of GCase and is in development for the treatment of PD, with GBA1-PD as the lead indication. The orally administered, brain-penetrant small molecule restores the function of GCase. In preclinical models of PD, GT-02287 restored GCase enzymatic function, reduced cellular stress in the endoplasmic reticulum, improved lysosomal function and mitochondrial function, reduced toxic glycosphingolipids, aggregated -synuclein, neuroinflammation and neuronal death, increased dopamine levels and improved motor function. Currently, the drug is in Phase I trial for the treatment of Parkinson's disease.
* NNI 362: Neuronascent
NNI-362 is Neuronascent's patented oral therapeutic aimed at reversing age-related disorders by producing new neurons to replace those lost in chronic neurodegenerative disorders and aging. NNI-362 has a unique mechanism of action (MOA) that provides safety and selectivity specifically for neuron regeneration, and was discovered through Neuronascent's proprietary phenotypic screening program using human neural progenitors. This MOA takes advantage of the neural progenitors already in the aged brain to promote new neurons and protect them from degeneration in chronic diseases of the aged, specifically Alzheimer's disease. New neuron regeneration from neural progenitors is a physiologic process in the brain, yet with aging and neurodegenerative disease there begins a pathological insufficiency in generating new neurons that survive to functionality. Thus, NNI-362 is aimed at reversing age-related neuron loss and restoring cognitive function in AD patients, and should also be useful for other disorders of the elderly, including Parkinson's disease and age-related hearing loss. Currently, the drug is in preclinical stage for the treatment of Parkinson's disease.
Explore groundbreaking therapies and clinical trials in the Parkinson's Disease Pipeline. Access DelveInsight's detailed report now! @ New Parkinson's Disease Drugs [https://www.delveinsight.com/sample-request/parkinsons-disease-pipeline-insights?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
The Parkinson's Disease Pipeline Report Provides Insights into-
* The report provides detailed insights about companies that are developing therapies for the treatment of Parkinson's Disease with aggregate therapies developed by each company for the same.
* It accesses the Different therapeutic candidates segmented into early-stage, mid-stage, and late-stage of development for Parkinson's Disease Treatment.
* Parkinson's Disease Companies are involved in targeted therapeutics development with respective active and inactive (dormant or discontinued) projects.
* Parkinson's Disease Drugs under development based on the stage of development, route of administration, target receptor, monotherapy or combination therapy, a different mechanism of action, and molecular type.
* Detailed analysis of collaborations (company-company collaborations and company-academia collaborations), licensing agreement and financing details for future advancement of the Parkinson's Disease market
Parkinson's Disease Companies
Annovis Bio, Cerevel Therapeutics, Roche, Inhibikase Therapeutics, Inc, Gain Therapeutics, Neuronascent, ANEW MEDICAL, Longevity Biotech, Minerva Neurosciences/Mitsubishi Tanabe Pharma Corporation, Tetraneuron, Kallyope, BlueRock Therapeutics LP, Neuraly, Peptron/Yuhan Corporation, Genentech, Kissei Pharmaceutical, Asklepios BioPharmaceutical, Alkahest, Modag, Intra-Cellular Therapies, Living Cell Technologies, MedImmune and others.
Parkinson's disease pipeline report provides the therapeutic assessment of the pipeline drugs by the Route of Administration. Products have been categorized under various ROAs, such as
* Intravenous
* Subcutaneous
* Oral
* Intramuscular
Parkinson's Disease Products have been categorized under various Molecule types such as
* Monoclonal antibody
* Small molecule
* Peptide
Unveil the future of Parkinson's Disease Treatment. Learn about new drugs, Parkinson's Disease Pipeline developments, and key companies with DelveInsight's expert analysis @ Parkinson's Disease Market Drivers and Barriers [https://www.delveinsight.com/sample-request/parkinsons-disease-pipeline-insights?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Scope of the Parkinson's Disease Pipeline Report
* Coverage- Global
* Parkinson's Disease Companies- Annovis Bio, Cerevel Therapeutics, Roche, Inhibikase Therapeutics, Inc, Gain Therapeutics, Neuronascent, ANEW MEDICAL, Longevity Biotech, Minerva Neurosciences/Mitsubishi Tanabe Pharma Corporation, Tetraneuron, Kallyope, BlueRock Therapeutics LP, Neuraly, Peptron/Yuhan Corporation, Genentech, Kissei Pharmaceutical, Asklepios BioPharmaceutical, Alkahest, Modag, Intra-Cellular Therapies, Living Cell Technologies, MedImmune and others.
* Parkinson's Disease Pipeline Therapies- VTX3232, Rasagiline, Safinamide Methanesulfonate, Levetiracetam, CTC-413, CVXL-0107, Levodopa, Rotigotine, Istradefylline 20 mg or 40 mg, IPX054 100 mg and others.
* Parkinson's Disease Therapeutic Assessment by Product Type: Mono, Combination, Mono/Combination
* Parkinson's Disease Therapeutic Assessment by Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
Get the latest on Parkinson's Disease Pipeline Therapies and clinical trials. Download DelveInsight's in-depth pipeline report today! @ Parkinson's Disease Companies, Key Products and Unmet Needs [https://www.delveinsight.com/sample-request/parkinsons-disease-pipeline-insights?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Table of Contents
* Introduction
* Executive Summary
* Parkinson's disease : Overview
* Pipeline Therapeutics
* Therapeutic Assessment
* Parkinson's Disease- DelveInsight's Analytical Perspective
* Late Stage Products (Phase III)
* Buntanetap: Annovis Bio
* Drug profiles in the detailed report.....
* Mid Stage Products (Phase II)
* Risvodetinib: Inhibikase Therapeutics, Inc
* Drug profiles in the detailed report.....
* Early Stage Products (Phase I)
* GT 02287: Gain Therapeutics
* Drug profiles in the detailed report.....
* Preclinical and Discovery Stage Products
* NNI 362: Neuronascent
* Drug profiles in the detailed report.....
* Inactive Products
* Parkinson's disease Key Companies
* Parkinson's disease Key Products
* Parkinson's disease - Unmet Needs
* Parkinson's disease - Market Drivers and Barriers
* Parkinson's disease - Future Perspectives and Conclusion
* Parkinson's disease Analyst Views
* Parkinson's disease Key Companies
* Appendix
About Us
DelveInsight is a leading healthcare-focused market research and consulting firm that provides clients with high-quality market intelligence and analysis to support informed business decisions. With a team of experienced industry experts and a deep understanding of the life sciences and healthcare sectors, we offer customized research solutions and insights to clients across the globe. Connect with us to get high-quality, accurate, and real-time intelligence to stay ahead of the growth curve.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Yash Bhardwaj
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=parkinson-disease-pipeline-drugs-2025-report-emerging-drugs-innovative-therapies-clinical-trial-updates-and-top-companies]
Phone: 09650213330
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: NV
Country: United States
Website: https://www.delveinsight.com/report-store/parkinsons-disease-pipeline-insight
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Parkinson Disease Pipeline Drugs 2025 Report: Emerging Drugs, Innovative Therapies, Clinical Trial Updates, and Top Companies here
News-ID: 4214604 • Views: …
More Releases from ABNewswire

Windy City Chimney Heroes Announces Expanded Service Areas Across the Greater Ch …
Windy City Chimney Heroes, a long-standing chimney company established in 1997, has expanded its service areas across the Greater Chicago region. The company now serves Chicago, Evanston, Oak Park, Naperville, Schaumburg, Cicero, Skokie, Berwyn, and Arlington Heights. Owner Wesley Cook says the expansion will help meet rising demand for reliable chimney repair and inspection services.
Windy City Chimney Heroes, a trusted chimney repair and maintenance provider founded in 1997, has announced…

Injury 2 Wellness Centers Promotes Wellness Care Through Ongoing Chiropractic Su …
As the year draws to a close and many individuals begin setting health goals for the new year, Injury 2 Wellness Centers is encouraging Georgia residents to prioritize wellness care through consistent chiropractic treatment. Far beyond addressing back or neck pain, chiropractic care can serve as a foundation for long-term physical health, improved function, and overall quality of life.
Decatur, GA - December 10, 2025 - As the year draws to…

Avalon Tree Services Reminds Atlanta Homeowners to Prioritize Winter Tree Care f …
As temperatures drop and trees enter their dormant season, Avalon Tree Services is reminding homeowners across Greater Atlanta that winter is one of the most important times to invest in proper tree care. Cold weather, ice accumulation, and seasonal storms can all take a toll on tree health and stability-making preventative maintenance essential for safety and long-term growth.
Atlanta, GA - December 10, 2025 - As temperatures drop and trees enter…
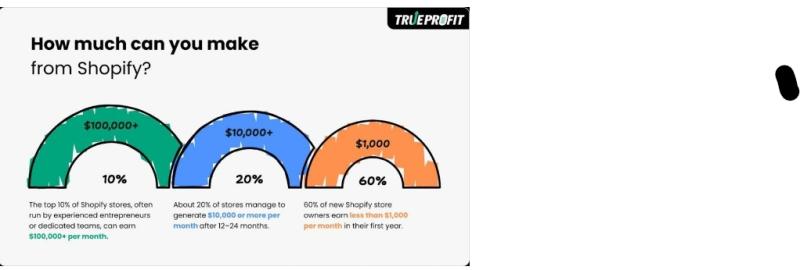
TrueProfit Shares 2026 Guidance for Shopify Startups to Focus on Profit First No …
As Shopify heads into 2026 with millions of active stores worldwide and continued growth in global ecommerce, TrueProfit - a Net Profit Analytics platform for Shopify merchants - is urging new founders to treat profitability as a starting requirement, not an afterthought.
With a low barrier to entry and a mature app ecosystem, Shopify remains one of the easiest ways to launch an online business. But TrueProfit's latest briefing on early-stage…
More Releases for Parkinson
Key Trends Reshaping the Wolff Parkinson White Syndrome Market: Technological Ad …
Stay ahead with our updated market reports featuring the latest on tariffs, trade flows, and supply chain transformations.
Wolff Parkinson White Syndrome Market Size Growth Forecast: What to Expect by 2025?
The market for Wolff Parkinson White syndrome has registered consistent growth over the recent past. The expectation is that it will expand from $1.17 billion in 2024 to $1.22 billion in 2025, reflecting a compound annual growth rate (CAGR) of 4.5%.…
Evolving Market Trends In The Anti-Parkinson Drugs Industry: Innovative Advancem …
The Anti-Parkinson Drugs Market Report by The Business Research Company delivers a detailed market assessment, covering size projections from 2025 to 2034. This report explores crucial market trends, major drivers and market segmentation by [key segment categories].
What Is the Expected Anti-Parkinson Drugs Market Size During the Forecast Period?
The anti-parkinson drugs market has seen significant growth in recent years, projected to increase from $10.37 billion in 2024 to $11.08 billion in…
What's Driving the Anti-Parkinson Drugs Market 2025-2034: Increasing Geriatric P …
What Are the Projections for the Size and Growth Rate of the Anti-Parkinson Drugs Market?
In recent times, the market size for anti-Parkinson drugs has experienced robust growth. The market, which is projected to rise from $10.37 billion in 2024 to $11.08 billion in 2025, boasts a compound annual growth rate (CAGR) of 6.9%. The past growth trend can be credited to an aging demographic, a surge in disease incidence, enhanced…
What's Driving the Anti-Parkinson Drugs Market 2025-2034: Increasing Geriatric P …
What Are the Projections for the Size and Growth Rate of the Anti-Parkinson Drugs Market?
In recent times, the market size for anti-Parkinson drugs has experienced robust growth. The market, which is projected to rise from $10.37 billion in 2024 to $11.08 billion in 2025, boasts a compound annual growth rate (CAGR) of 6.9%. The past growth trend can be credited to an aging demographic, a surge in disease incidence, enhanced…
Wolff Parkinson White Syndrome Treatment Market Overview 2024-2033
The Business Research Company has recently revised its global market reports, now incorporating the most current data for 2024 along with projections extending up to 2033.
Wolff Parkinson White Syndrome Global Market Report 2024 by The Business Research Company offers comprehensive market insights, empowering businesses with a competitive edge. It includes detailed estimates for numerous segments and sub-segments, providing valuable strategic guidance.
The Market Size Is Expected To Reach $1.43 billion…
Anti-Parkinson Drugs Market Overview
Exhibiting robust growth, the anti-parkinson drugs market is set to escalate from $9.73 billion in 2023 to $10.43 billion in 2024, with a notable Compound Annual Growth Rate (CAGR) of 7.2%. The trajectory continues on an upward trend, with an anticipated market size of $13.36 billion by 2028, sustaining a robust CAGR of 6.4%.
Increasing Geriatric Population Driving Demand:
The burgeoning geriatric population, coupled with a surge in Parkinson's disease cases,…
