Press release
South East Asia Cancer Immunotherapy Market Valuation to Reach USD 10,384.0 Million by 2033 - Industry Expanding at a CAGR of 8.83%
Market Overview:According to IMARC Group's latest research publication, "South East Asia Cancer Immunotherapy Market Size, Share, Trends and Forecast by Therapy Type, Application, End User, Country, and Company, 2025-2033", the South East Asia cancer immunotherapy market size reached USD 4,847.9 Million in 2024. Looking forward, the market is expected to reach USD 10,384.0 Million by 2033, exhibiting a growth rate (CAGR) of 8.83% during 2025-2033.
This detailed analysis primarily encompasses industry size, business trends, market share, key growth factors, and regional forecasts. The report offers a comprehensive overview and integrates research findings, market assessments, and data from different sources. It also includes pivotal market dynamics like drivers and challenges, while also highlighting growth opportunities, financial insights, technological improvements, emerging trends, and innovations. Besides this, the report provides regional market evaluation, along with a competitive landscape analysis.
Grab a sample PDF of this report: https://www.imarcgroup.com/south-east-asia-cancer-immunotherapy-market/requestsample
Our report includes:
Market Dynamics
Market Trends and Market Outlook
Competitive Analysis
Industry Segmentation
Strategic Recommendations
Growth Factors in the South East Asia Cancer Immunotherapy Market
Rising Cancer Burden Driving Demand for Advanced Treatment Solutions
South East Asia is experiencing a significant surge in cancer cases, and the numbers tell a compelling story. The region, home to over 690 million people, is witnessing cancer emerge as a leading cause of mortality and morbidity. What makes this trend particularly noteworthy is the convergence of multiple factors-aging demographics, urbanization, and lifestyle changes are creating perfect conditions for market expansion. The shift in disease patterns is unmistakable. Lung cancer, breast cancer, and colorectal cancer cases are climbing steadily across the region, driven by factors like increased smoking rates in certain populations, dietary changes, and environmental factors. Healthcare systems across Indonesia, Thailand, Singapore, Philippines, Vietnam, and Malaysia are responding by prioritizing oncology services and integrating advanced treatment modalities.
Traditional cancer treatments like chemotherapy and radiation have been mainstays for decades, but they come with significant limitations-severe side effects, non-specific targeting, and variable effectiveness. This creates tremendous opportunity for immunotherapy, which offers a fundamentally different approach by mobilizing the body's own immune system. The appeal is straightforward: immunotherapy can target cancer cells with precision while leaving healthy tissue largely unaffected, potentially leading to durable responses with manageable side effects. For patients dealing with melanoma, certain leukemias, and advanced lung cancers, immunotherapy represents hope where conventional options have exhausted their potential. Healthcare providers are increasingly incorporating these treatments into standard protocols, recognizing that early adoption gives their patients access to potentially life-extending therapies.
Government Support and Healthcare Infrastructure Investment Accelerating Access
What's transforming the landscape is how governments across South East Asia are stepping up their commitment to cancer care. The WHO South-East Asia Regional Strategy for comprehensive cancer prevention and management, launched for implementation through 2030, signals serious institutional backing for cancer control initiatives. This isn't just policy documentation-it's translating into tangible support through research funding, streamlined regulatory frameworks, and public awareness campaigns that destigmatize cancer and encourage early detection. Thailand's Ministry of Public Health has pioneered the "Cancer Anywhere" project, a groundbreaking initiative that ensures cancer patients under the Universal Coverage Scheme can access multimodality treatments at any public hospital, regardless of where they're registered.
This addresses a critical barrier-geographic access-that has historically prevented rural and underserved populations from receiving advanced care. The project represents exactly the kind of pragmatic policy innovation that makes sophisticated treatments like immunotherapy accessible beyond major metropolitan centers. Singapore continues to position itself as a regional hub for advanced cancer care, with cutting-edge facilities and clinical trial infrastructure that attracts international pharmaceutical companies and research institutions.
The country's regulatory environment facilitates faster approval pathways for innovative therapies, creating opportunities for patients to access new immunotherapy agents earlier. Across the region, investments in healthcare infrastructure are expanding-new cancer centers, specialized oncology departments, and enhanced laboratory capabilities are being established. These facilities aren't just treating patients with existing therapies; they're participating in clinical trials, contributing to research, and training the next generation of oncologists who understand immunotherapy protocols. The infrastructure buildout creates a virtuous cycle: better facilities attract research investment, which generates clinical data, which supports regulatory approvals, which increases patient access.
Pharmaceutical Partnerships and Research Collaborations Driving Innovation
The South East Asia cancer immunotherapy market is benefiting enormously from strategic partnerships between pharmaceutical companies, research institutions, and healthcare providers. These collaborations are accelerating both the development of new immunotherapeutic agents and their path to market. What makes this ecosystem particularly dynamic is how it combines global pharmaceutical expertise with regional clinical insights and patient populations. Major pharmaceutical players are establishing research hubs and clinical trial sites across the region, recognizing that South East Asian populations offer unique opportunities for studying cancer immunotherapy effectiveness across diverse genetic backgrounds and cancer profiles. Lion TCR, for instance, has established what they describe as the world's largest HBV-specific T-cell receptor library, covering approximately 80% of populations in China, East Asia, and South East Asia.
This kind of regionally-focused research addresses specific disease patterns prevalent in Asian populations. The collaborative model extends beyond pure research into commercialization and access programs. Pharmaceutical companies are working with local distributors, hospital systems, and insurance providers to develop pricing structures and reimbursement pathways that make immunotherapy economically viable. This matters tremendously because checkpoint inhibitors, monoclonal antibodies, and other immunotherapy agents carry significant price tags-creating access barriers if not addressed through innovative financing solutions.
Academic medical centers across the region are participating in international clinical trials, contributing patient data and clinical outcomes that inform global treatment guidelines. Singapore's research institutions, Thailand's university hospitals, and Malaysia's specialized cancer centers are generating real-world evidence about immunotherapy effectiveness in South East Asian patient populations. This evidence base supports regulatory submissions, helps refine treatment protocols, and builds confidence among healthcare providers about integrating immunotherapy into practice. The partnerships also facilitate knowledge transfer-training programs, symposia, and clinical exchanges ensure oncologists across the region stay current with rapidly evolving immunotherapy science and treatment protocols.
Key Trends in the South East Asia Cancer Immunotherapy Market
Monoclonal Antibodies Leading Therapy Type Adoption
The breakdown by therapy type reveals clear preferences in clinical practice. Monoclonal antibodies represent the most established category within cancer immunotherapy, and their adoption across South East Asia reflects both their clinical track record and growing familiarity among oncologists. These engineered antibodies work by binding to specific targets on cancer cells or immune cells, either marking cancer cells for destruction or blocking signals that allow cancer to evade immune surveillance. What makes monoclonal antibodies particularly attractive for healthcare providers is their well-documented efficacy across multiple cancer types and relatively predictable safety profiles compared to earlier immunotherapy approaches. Checkpoint inhibitors are experiencing rapid uptake, representing one of the most exciting developments in oncology.
These drugs work by releasing the "brakes" on immune system cells, allowing them to recognize and attack cancer more effectively. The class includes PD-1 inhibitors, PD-L1 inhibitors, and CTLA-4 inhibitors-each working through slightly different mechanisms but sharing the common goal of enhancing immune response against tumors. The clinical data supporting checkpoint inhibitors has been compelling, with some patients achieving durable responses that extend survival significantly beyond what conventional therapy could deliver. Cancer vaccines and immunomodulators represent emerging categories with tremendous potential but still developing clinical adoption.
Cancer vaccines attempt to stimulate immune responses against specific tumor antigens, essentially training the immune system to recognize cancer cells as foreign threats. While more experimental than monoclonal antibodies or checkpoint inhibitors, they represent an important frontier in personalized cancer treatment. The therapy type distribution matters for market development because it influences everything from regulatory approvals to physician training to reimbursement decisions. Healthcare systems need to develop protocols for administering these different modalities, managing side effects, and determining which patients are likely to benefit most from each approach.
Hospital and Cancer Research Centers Dominating Treatment Delivery
When examining end users, hospitals emerge as the primary delivery channel for cancer immunotherapy across South East Asia. This concentration makes intuitive sense given the complexity of immunotherapy administration and monitoring requirements. These treatments typically require intravenous infusion in clinical settings with appropriate emergency response capabilities, regular monitoring for immune-related adverse events, and access to specialized oncology expertise. Major hospitals and academic medical centers have the infrastructure, trained personnel, and multidisciplinary teams necessary to deliver immunotherapy safely and effectively. Cancer research centers represent another critical end user segment, serving dual roles as treatment facilities and sites for clinical investigation.
These specialized institutions are often where the newest immunotherapy protocols are evaluated, where combination therapies are tested, and where oncologists gain expertise with emerging treatment approaches. For patients enrolled in clinical trials, research centers provide access to experimental immunotherapy agents not yet available through standard care-potentially life-extending opportunities for those who have exhausted conventional options. The research center network across South East Asia is expanding, with institutions in Singapore, Thailand, and Malaysia establishing themselves as regional leaders in oncology research. Clinics and smaller healthcare facilities represent a growing segment, particularly as immunotherapy protocols become more standardized and certain agents move toward broader adoption.
Specialized oncology clinics in urban areas are beginning to offer immunotherapy options, making treatment more geographically accessible and potentially reducing the burden on major hospital systems. However, the complexity and cost of immunotherapy mean that smaller facilities face real challenges in adoption-they need trained staff, appropriate storage facilities for biological agents, and established referral pathways for managing complications. The end user distribution affects market accessibility profoundly. Concentration in major hospitals and research centers means patients in rural areas often must travel significant distances for treatment, creating barriers related to cost, time, and social support. Expanding capability across more healthcare settings represents a key opportunity for market growth, but requires substantial investment in training, infrastructure, and supply chain development.
● Indonesia and Thailand Leading Regional Market Development
The country-level analysis reveals distinct patterns in market development across South East Asia. Indonesia, as the region's most populous nation with over 270 million people, represents enormous market potential. The sheer size of the population, combined with rising cancer incidence rates, creates substantial demand for advanced treatment options. Indonesia's healthcare system has been expanding oncology services, though significant geographic disparities remain between urban centers like Jakarta and more remote provinces. The government has prioritized cancer control initiatives, recognizing the growing disease burden and working to improve access to modern treatments. Thailand has positioned itself as a healthcare destination for the region, known for quality medical services at competitive costs.
The country's National Cancer Control Program demonstrates systematic commitment to comprehensive cancer care, and initiatives like the "Cancer Anywhere" project show innovative approaches to access barriers. Thai hospitals and cancer centers are active participants in international clinical trials, giving Thai patients earlier access to novel immunotherapy agents. The country's medical tourism industry also means healthcare facilities are accustomed to delivering sophisticated treatments to international standards. Singapore stands out for its advanced healthcare infrastructure and position as a regional innovation hub. The city-state's research institutions, regulatory framework, and clinical expertise make it a natural entry point for pharmaceutical companies introducing new immunotherapy products to South East Asian markets. Singapore's patients often get first access to cutting-edge treatments, and the country's outcomes data influences treatment decisions across the region.
Philippines, Vietnam, and Malaysia each contribute significantly to market growth, driven by expanding healthcare systems, rising middle-class populations with greater health insurance coverage, and increasing awareness of advanced cancer treatment options. Vietnam's healthcare sector has been modernizing rapidly, with major cities like Ho Chi Minh City and Hanoi developing specialized oncology capabilities. Malaysia's healthcare system serves both domestic patients and medical tourists from neighboring countries, with established cancer centers offering comprehensive treatment options including immunotherapy.
The Philippines faces challenges related to healthcare financing and geographic dispersion across thousands of islands, but its large population and growing economy create substantial long-term market opportunity. Understanding these country-level dynamics matters because market development strategies need to address specific regulatory environments, reimbursement systems, healthcare infrastructure capabilities, and patient populations in each market. What works in Singapore's tightly integrated healthcare system may require substantial adaptation for Indonesia's more fragmented landscape or Thailand's public health-focused approach.
Ask analyst of customized report: https://www.imarcgroup.com/request?type=report&id=20504&flag=E
Leading Companies Operating in the South East Asia Cancer Immunotherapy Market:
The market features participation from major global pharmaceutical companies and biotechnology firms with established immunotherapy portfolios. These organizations are engaged in research, development, clinical trials, and commercialization of immunotherapeutic agents across the region.
South East Asia Cancer Immunotherapy Market Report Segmentation:
Breakup by Therapy Type:
● Monoclonal Antibodies
● Cancer Vaccines
● Checkpoint Inhibitors
● Immunomodulators
● Others
Breakup by Application:
● Lung Cancer
● Breast Cancer
● Colorectal Cancer
● Melanoma
● Prostate Cancer
● Head and Neck Cancer
● Others
Breakup by End User:
● Hospitals
● Cancer Research Centers
● Clinics
● Others
Country Insights:
● Indonesia
● Thailand
● Singapore
● Philippines
● Vietnam
● Malaysia
● Others
Research Methodology:
The report employs a comprehensive research methodology, combining primary and secondary data sources to validate findings. It includes market assessments, surveys, expert opinions, and data triangulation techniques to ensure accuracy and reliability.
Note: If you require specific details, data, or insights that are not currently included in the scope of this report, we are happy to accommodate your request. As part of our customization service, we will gather and provide the additional information you need, tailored to your specific requirements. Please let us know your exact needs, and we will ensure the report is updated accordingly to meet your expectations.
About Us:
IMARC Group is a global management consulting firm that helps the world's most ambitious changemakers to create a lasting impact. The company provides a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No: (D) +91-120-433-0800
United States: +1-201-971-6302
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release South East Asia Cancer Immunotherapy Market Valuation to Reach USD 10,384.0 Million by 2033 - Industry Expanding at a CAGR of 8.83% here
News-ID: 4211472 • Views: …
More Releases from IMARC Group
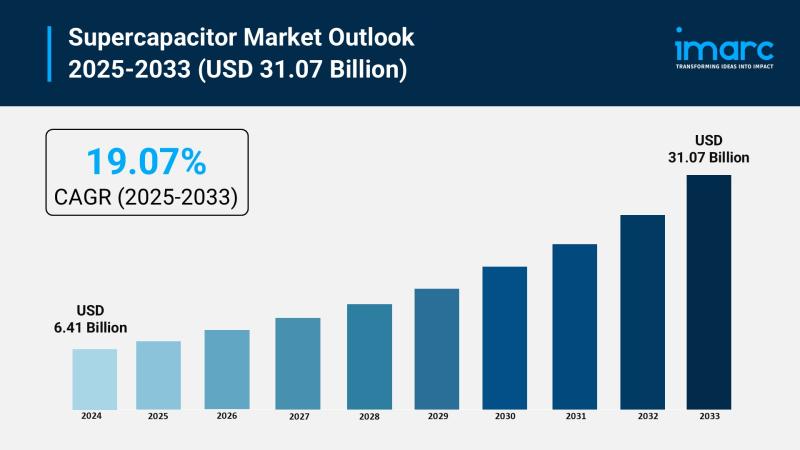
Supercapacitor Market Size to Reach $31.07B by 2033: Trends & Opportunities
Market Overview:
The supercapacitor market is experiencing rapid growth, driven by electrification of automotive systems, renewable energy and grid stabilization, and expansion of industrial automation and robotics. According to IMARC Group's latest research publication, "Supercapacitor Market Size, Share, Trends and Forecast by Product Type, Module Type, Material Type, End Use Industry, and Region, 2025-2033", the global supercapacitor market size was valued at USD 6.41 Billion in 2024. Looking forward, IMARC Group…
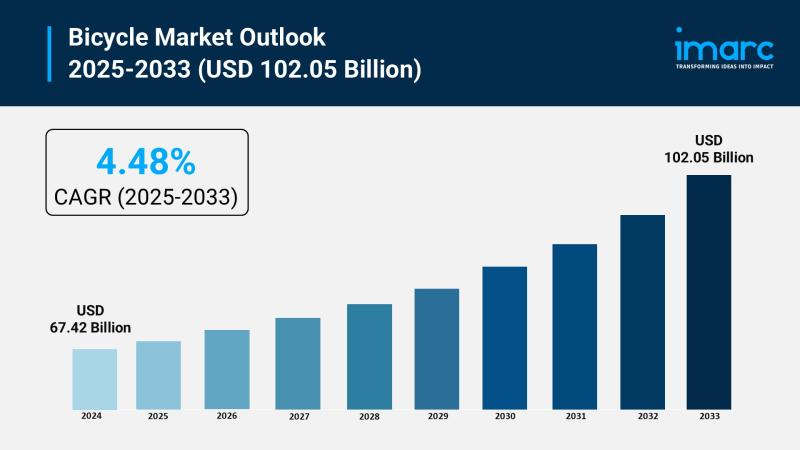
Bicycle Market Size to Surpass $102.05B by 2033: Growth & Insights
Market Overview:
The bicycle market is experiencing rapid growth, driven by global expansion of cycling infrastructure, rising health consciousness and preventative wellness, and technological advancements in e-bike propulsion. According to IMARC Group's latest research publication, "Bicycle Market Size, Share, Trends and Forecast by Type, Technology, Price, Distribution Channel, End User, and Region, 2025-2033", The global bicycle market size was valued at USD 67.42 Billion in 2024. Looking forward, IMARC Group estimates…
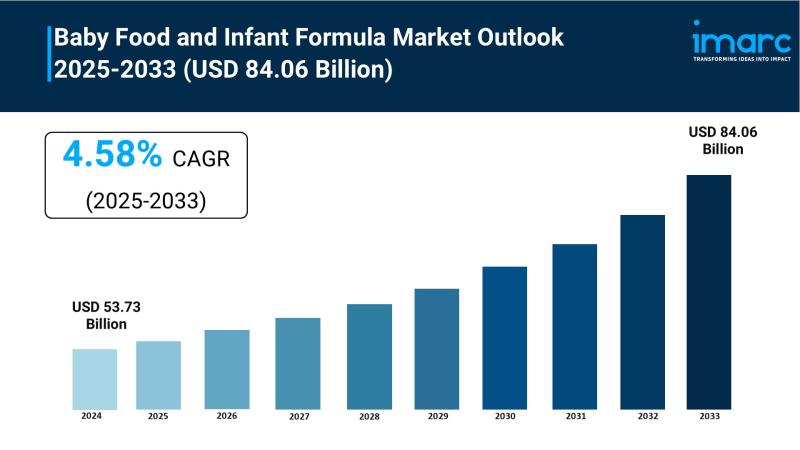
Baby Food and Infant Formula Market to Reach USD 84.06 Billion by 2033, Growing …
Market Overview:
The Baby Food and Infant Formula Market is experiencing steady expansion, driven by Increasing Awareness of Nutritional Needs for Infants, Rising Number of Working Women, and Technological Advancements and Product Innovation. According to IMARC Group's latest research publication, "Baby Food and Infant Formula Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2025-2033", The global baby food and infant formula market size reached USD 53.73 Billion in 2024.…
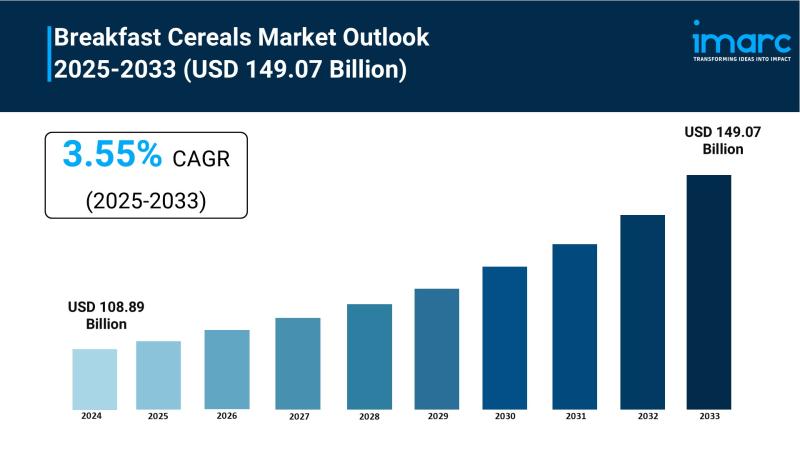
Breakfast Cereals Market to Reach USD 149.07 Billion by 2033, Growing at a CAGR …
Market Overview:
The Breakfast Cereals Market is experiencing rapid growth, driven by Health and Wellness Awareness, Busy Lifestyles and On-the-Go Demand and Rising Disposable Incomes and Global Market Expansion . According to IMARC Group's latest research publication, "Breakfast Cereals Market : Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2025-2033", The global breakfast cereals market size was valued at USD 108.89 Billion in 2024. Looking forward, IMARC Group estimates…
More Releases for Asia
Asia Private Equity Firm, Asia Private Equity Management, Asia Private Equity Se …
The private equity market in China has been rapidly growing in recent years. Private equity (PE) refers to the purchase of shares in a company that are not publicly traded on a stock exchange. PE firms typically target companies that are undervalued or in need of capital for growth, and aim to improve the company's operations and financial performance before selling it at a higher value.
https://boomingfaucet.com/
Asia Private Equity Consulting
E-mail:nolan@pandacuads.com
In China,…
South East Asia Business Jet Market And Top Key Players are Asia Corporate Jet, …
By 2022, the South East Asia Business Jet Markets estimated to reach US$ XX Mn, up from US$ XX Mn in 2016, growing at a CAGR of XX% during the forecast period. The Global Business Jet Market, currently at 21 million USD, contributes the highest share in the market and is poised to grow at the fastest rate in the future. The three broad categories of business jets are Small,…
LIXIL Asia Presents Asia Pacific Property Awards
Through its power brands GROHE and American Standard, LIXIL Asia signs a three-year deal to become the Headline Sponsor of the Asia Pacific Property Awards from 2019 until 2022.
23rd January 2019: The International Property Awards, first established in 1993, are open to residential and commercial property professionals from around the globe. They celebrate the highest levels of achievement by companies operating within the architecture, interior design, real estate and property…
PEOPLEWAVE WINS ASIA TECH PODCAST PITCHDECK ASIA 2019 AWARDS
15 January 2019, Singapore – Peoplewave, Asia’s leading data-driven HR technology company, won the Asia Tech Podcast (ATP) Pitchdeck Asia 2019 Awards, being awarded “Startup Most Likely to Succeed in 2019".
The 2019 Pitchdeck Asia Awards is an opportunity for the Asian Startup Ecosystem to shine a spotlight on some of its best startups. The awards were decided by a public vote. More than 7,200 votes were cast by registered LinkedIn…
Undersea Defence Technology Asia, UDT Asia 2011
Latest Military Diving Technologies featured in UDT Asia
Equipping Asia’s navies with the latest diving technology for asymmetric warfare and
operations
SINGAPORE, 17 October 2011 - Naval diving and underwater special operations is a field that is
seeing increased attention and investment amongst navies in Asia. Units such as the Indonesian Navy‟s KOPASKA, the Republic of Singapore Navy‟s Naval Diving Unit (NDU), the Royal Malaysian Navy‟s PASKAL are increasingly utilising specialised equipment for conducting…
Asia Diligence – Specialist Investigative Due Diligence for Asia & Beyond
Asia Diligence today announced the opening of its European Customer Services office in the United Kingdom. The office is to be managed by Steve Fowler and will focus on providing services to Asia Diligence’s European customers. Asia Diligence is also planning to open a US office in the near future, which will provide customer service to its US and North American clients.
Asked to comment on the move, Luke Palmer, the…
