Press release
Biosimilars Market to Reach USD 125.9 Billion by 2035, Driven by mAb Adoption, Manufacturing Scale-Up, and Digital-Enabled Patient Management
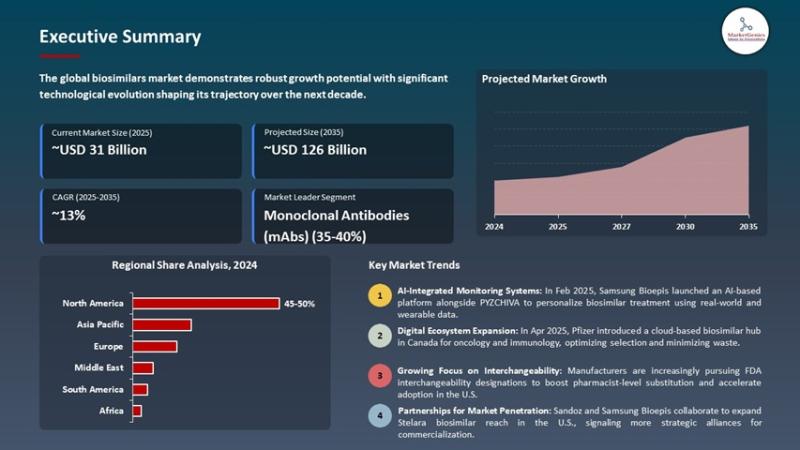
Biosimilars Market to Reach USD 125.9 Billion by 2035, Driven by mAb Adoption, Manufacturing Scale-Up, and Digital-Enabled Patient
This rapid expansion is driven by patent expiries on blockbuster biologics, increased payer and hospital adoption of lower-cost alternatives, and rising biosimilar availability across oncology, immunology, and endocrinology.
MarketGenics positions biosimilars as a strategic lever for sustainable healthcare, enabling broad access to biologic therapies while creating new service and digital-health adjacencies that support adherence, safety monitoring, and value-based care.
Get the Detailed Industry Analysis (including the Table of Contents, List of Figures, and List of Tables) - from the Biosimilars Market Research Report: https://marketgenics.co/press-releases/biosimilars-market-04169
Recent Developments Shaping the Market
Samsung Bioepis launches PYZCHIVA with AI monitoring (Feb 2025)
Samsung Bioepis introduced PYZCHIVA (a biosimilar to ustekinumab/Stelara) in the U.S., paired with an AI-enabled real-time monitoring platform that collects RWD and wearable data to personalize dosing and safety surveillance.
Pfizer expands biosimilar portfolio with IXIFI in Canada (Apr 2025)
Pfizer launched IXIFI (infliximab biosimilar) in Canada and rolled out an AI-based Biosimilar Management Hub for oncology and immunology providers to optimize treatment selection and inventory planning.
Manufacturing scale-ups and contract manufacturing momentum
Global CMOs and biopharma (Amgen, Biocon, Celltrion) have announced capacity expansions and modular bioreactor investments to reduce COGS and accelerate time-to-market for high-value mAb biosimilars.
Biosimilars Market Forecast 2035
The market is set to create an incremental opportunity of USD 94.4 billion between 2025 and 2035.
North America will remain the largest and most lucrative market because of strong payer reforms, physician familiarity, and active substitution policies in some jurisdictions.
Europe will sustain high uptake driven by tendering programs and established biosimilar pathways.
Asia-Pacific will be the fastest-growing region as local manufacturers scale capacity, governments push cost containment, and access expands in China, India, Korea and Southeast Asia.
By 2035, biosimilars will be embedded across standard treatment algorithms-lowering cost of care, increasing patient access to biologics, and enabling health systems to reallocate savings to innovative therapies.
To know more about the Biosimilars Market - Download our Sample Report: https://marketgenics.co/download-report-sample/biosimilars-market-04169
Key Drivers, Challenges, and Opportunities
Driver - Patent cliffs and cost-containment pressure
Expiries of major biologic patents open large addressable markets. Payers and hospitals favor biosimilars to reduce drug spend, driving rapid formulary inclusion-especially for high-cost oncology and autoimmune biologics.
Restraint - Complex development, regulatory hurdles and interchangeability debates
Unlike small-molecule generics, biosimilars require costly analytical comparability, clinical evidence, and robust manufacturing controls. Interchangeability/substitution policies vary by market and can slow uptake where pharmacy-level substitution is restricted.
Opportunity - Value-added services & digital patient hubs
Providers who combine biosimilars with digital adherence tools, RWD safety monitoring, and AI-driven treatment-selection platforms can capture premium margins and accelerate clinician confidence. Examples: AI monitoring platforms paired with PYZCHIVA and Pfizer's management hub.
Key Trend - Manufacturing Scale-Up & Tendering Economics
Large biosimilar wins increasingly hinge on manufacturing cost efficiency and tender-based pricing. Modular biomanufacturing, single-use systems, and regional CMO networks are compressing COGS and enabling more aggressive pricing-particularly in public tender markets in Europe and Latin America.
Buy Now: https://marketgenics.co/buy/biosimilars-market-04169
Segmental Insights
mAbs Lead (~36% share in 2025)
Monoclonal antibody biosimilars dominate due to wide therapeutic coverage (oncology, RA, IBD) and high list prices for originators-making savings substantial and uptake rapid where policies support substitution.
Recombinant proteins & insulins
Recombinant hormones, insulin analogs and G-CSF biosimilars form growing mid-value segments, especially as diabetic care and supportive oncology therapy volumes rise.
Regional Highlights
North America: Market leader-high pricing pressure, growing payer programs, and increasing physician acceptance; rapid commercialization of complex biosimilars.
Europe: Early biosimilar adopters with mature tender frameworks and high penetration in hospital formularies.
Asia-Pacific: Fastest expansion-local manufacturing scale, price sensitivity, and public programs driving adoption.
Middle East, Africa & Latin America: Selective uptake tied to tender policies, local licensing, and access programs.
Competitive Landscape
The biosimilars market is moderately consolidated, with the top players controlling significant share but with active regional challengers.
Tier-1 Leaders: Pfizer, Amgen, Novartis (Sandoz), Samsung Bioepis, Biocon Biologics.
Tier-2 Players: Celltrion, Fresenius Kabi, Mylan/Viatris, Celltrion, Teva (regional playbooks).
Innovators & Niche: Xbrane, Kashiv BioSciences, regional biotechs focused on cost-optimized pipelines.
Competition centers on manufacturing scale, COGS reduction, regulatory evidence, tender execution, and bundled digital services.
Get a preview of our Biosimilars Market Playbook - your guide to GTM strategy, competitive intelligence, supplier dynamics, and Consumer Behavior Analysis: https://marketgenics.co/playbook/biosimilars-market-04169
Future Outlook
By 2035 the biosimilars market will be characterized by:
Wider therapeutic coverage (mAbs, fusion proteins, insulin analogs) and deeper penetration across hospitals and outpatient clinics;
Lowered treatment costs that enable broader population access to biologics;
Integrated digital ecosystems (monitoring, adherence, RWE) that de-risk substitution and support payer value arguments;
Regional manufacturing hubs reducing lead times and enabling competitive pricing.
These forces will position biosimilars as a major driver of healthcare affordability-capturing a share of a USD 125.9 billion market by 2035.
Prominent Companies Operating in the Global Biosimilars Market
Amgen, Novartis (Sandoz), Pfizer, Samsung Bioepis, Biocon Biologics, Celltrion, Fresenius Kabi, Viatris (Mylan), Teva, Xbrane, Kashiv BioSciences, and other global/regional manufacturers.
About Us
MarketGenics is a global market research and management consulting company empowering decision makers across healthcare, technology, and policy domains. Our mission is to deliver granular market intelligence combined with strategic foresight to accelerate sustainable growth.
We support clients across strategy development, product innovation, healthcare infrastructure, and digital transformation.
Contact:
Mr. Debashish Roy
MarketGenics India Pvt. Ltd.
800 N King Street, Suite 304 #4208, Wilmington, DE 19801, United States
USA: +1 (302) 303-2617
Email: sales@marketgenics.co
Website: https://marketgenics.co
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Biosimilars Market to Reach USD 125.9 Billion by 2035, Driven by mAb Adoption, Manufacturing Scale-Up, and Digital-Enabled Patient Management here
News-ID: 4205260 • Views: …
More Releases from MarketGenics India Pvt. Ltd.
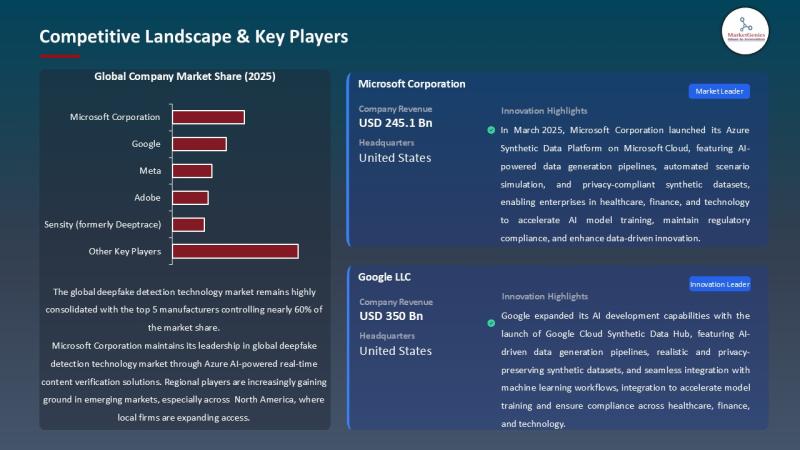
APAC Deepfake Detection Market Accelerates as Governments Tighten Digital Trust …
A Market Transforming How the World Verifies Reality
The global deepfake detection technology market, valued at USD 0.6 billion in 2025, is positioned to accelerate at a powerful 37.2% CAGR, reaching USD 15.1 billion by 2035.
This growth is driven by one undeniable truth:
Synthetic media is reshaping the threat landscape faster than humans can recognize it.
Deepfake detection technologies now determine:
How newsrooms verify breaking content
How financial institutions prevent identity-spoofing
How governments protect election integrity
How…
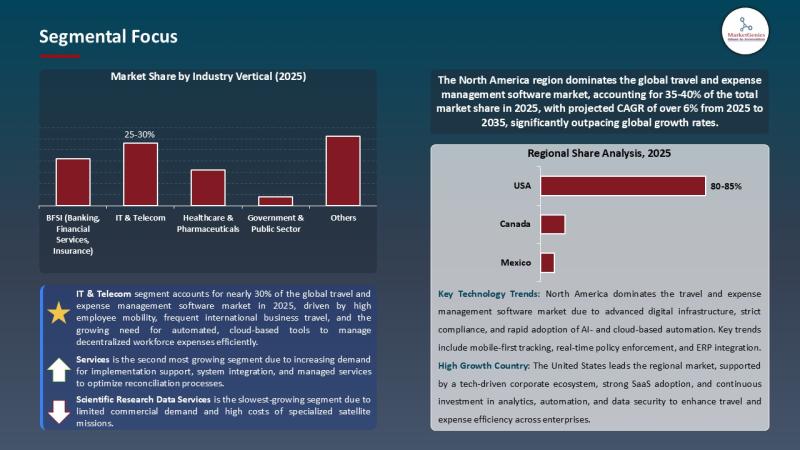
Travel & Expense Management Software Market Signals a Digital Pivot | AI, Cloud …
The Travel and Expense Management (TEM) Market Crossroads | A Sector Accelerating, Repricing Efficiency, and Redrawing the Corporate Spend Map
(Is TEM a Back-Office Tool-or the Operating System of the Next Enterprise Economy?)
For years, the travel and expense management software market lived in the administrative shadows-handed off to finance teams, constrained by spreadsheets, and dismissed as a routine cost-control tool. But the numbers now tell a radically different story.
In 2025, the…
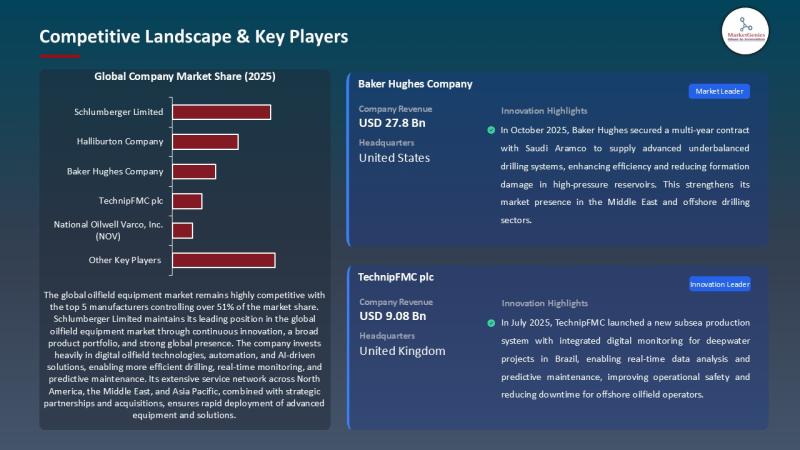
Oilfield Equipment Market hits USD 116.2B in 2025 and grows to USD 156.5B by 203 …
Oilfield Equipment Market | The $156.5B Hardware Backbone of the Global Energy System
Every headline loves clean energy. Yet the global energy mix still demands a brutal truth: oil and gas remain the world's primary supply of heat, mobility, and petrochemicals - and the machines that drill, lift, complete, and produce hydrocarbons continue to define industrial capability.
That's why the Oilfield Equipment Market remains a strategic industry - not a relic.
In 2025,…

Machine Tools Market 2025-2035 | USD 109.9B Growth, CNC & Automation Trends
Machine Tools Market | The $109.9B Intelligence Engine of Global Manufacturing
Factories don't work without machine tools. They shape, cut, drill, grind, and define the physical world around us. Yet most end-products - cars, aircraft parts, electronics housings, surgical devices - never reveal the precision machinery behind them.
The Machine Tools Market is the invisible infrastructure that turns digital models into physical reality.
In 2025, the global Machine Tools Market stands at USD…
More Releases for Biosimilar
Interchangeable Biosimilar Humira Market Share Driven by Biologic Therapy Adopti …
Interchangeable Biosimilar Humira Market
The global market for Interchangeable Biosimilar Humira was valued at US$ million in the year 2024 and is projected to reach a revised size of US$ million by 2031, growing at a CAGR of %during the forecast period
View sample report
https://reports.valuates.com/request/sample/QYRE-Auto-33I15005/Global_Interchangeable_Biosimilar_Humira_Market_Research_Report_2023
The Interchangeable Biosimilar Humira Market is experiencing significant market growth as healthcare providers and patients increasingly adopt biosimilar therapies for autoimmune and inflammatory conditions. Market trends indicate rising…
Key Trend Reshaping the Biosimilar Monoclonal Antibodies Market in 2025: Advance …
What Are the Projections for the Size and Growth Rate of the Biosimilar Monoclonal Antibodies Market?
In recent times, the biosimilar monoclonal antibodies sector has experienced a swift expansion. The market size, which stands at $8.04 billion in 2024, is projected to climb to $9.25 billion in 2025, marking a compound annual growth rate (CAGR) of 15.1%. Factors such as expired patents, an increased understanding of biosimilars, governmental strategies, heightened financial…
Key Trend Reshaping the Biosimilar Monoclonal Antibodies Market in 2025: Advance …
What Are the Projections for the Size and Growth Rate of the Biosimilar Monoclonal Antibodies Market?
In recent times, the biosimilar monoclonal antibodies sector has experienced a swift expansion. The market size, which stands at $8.04 billion in 2024, is projected to climb to $9.25 billion in 2025, marking a compound annual growth rate (CAGR) of 15.1%. Factors such as expired patents, an increased understanding of biosimilars, governmental strategies, heightened financial…
Biosimilar Market Treating More for Less: The Booming Infliximab Biosimilar Mark …
Infliximab Biosimilar Market worth $ XX Million by 2030 - Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Infliximab Biosimilar Market- by Application (Crohn's Disease, Psoriatic Arthritis, Rheumatoid Arthritis, Ulcerative Colitis, Ankylosing Spondylitis, Plaque Psoriasis and Others), End User (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy and Other Direct Distribution Channels), Trends, Industry Competition Analysis, Revenue and Forecast To 2030."
Get…
Biosimilar Monoclonal Antibodies Market
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the " "Global Biosimilar Monoclonal Antibodies Market by Product (infliximab, trastuzumab, rituximab, adalimumab, bevacizumab, cetuximab, ranibizumab, denosumab, eculizumab, and other pipeline products), Indication (oncology, inflammatory & autoimmune disorders, chronic diseases, blood disorders, and other indications), Clinical Trial/Pipeline Analysis, Future Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
The Biosimilar Monoclonal Antibodies Market Size is valued at 5.02…
Infliximab Biosimilar Insight, 2022 | DelveInsight
DelveInsight's, "Infliximab Biosimilar Insight, 2022" report provides comprehensive insights about 35+ companies and 45+ marketed and pipeline drugs in Infliximab Biosimilars landscape. It covers the marketed and pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Interested to know more about the functioning of…