Press release
Clinical Trial Services Market to Reach USD 135 Billion by 2035, Driven by AI, Decentralized Trials, and Sustainability-Centered Operations
Clinical Trial Services Market to Reach USD 135 Billion by 2035, Driven by AI, Decentralized Trials, and Sustainability-Centered O
MarketGenics, a leading research firm, identifies clinical trial services as a critical enabler of global drug development, underpinning faster execution, broader patient access, and improved compliance in an era of growing therapeutic complexity.
Get the Detailed Industry Analysis (including the Table of Contents, List of Figures, and List of Tables) - from the Clinical Trial Services Market Research Report: https://marketgenics.co/press-releases/clinical-trial-services-market-42377
Recent Developments Shaping the Market
Labcorp Expands AI-Powered Pathology Capabilities
In May 2025, Labcorp launched new NGS panels for hematologic malignancies and expanded its digital pathology platform with AI-driven infrastructure, enabling scalable companion diagnostics and accelerating oncology trial development.
IQVIA Deploys Custom AI Solutions for Research Workflows
In June 2025, IQVIA introduced agentic AI tools leveraging NVIDIA platforms to automate workflows such as target identification, clinical data review, and literature analysis, significantly boosting efficiency in trial execution.
Parexel Partners with Palantir on Data Management
In early 2025, Parexel integrated Palantir's AI-powered platform into its clinical trial services, optimizing data logistics and patient matching to shorten timelines and improve enrollment accuracy.
Clinical Trial Services Market Forecast 2035
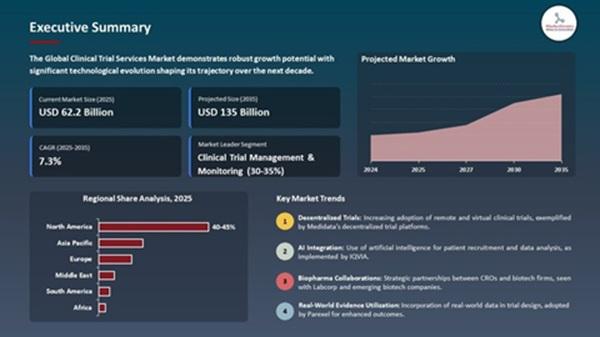
The market is set to create an incremental opportunity of USD 72.8 billion between 2025 and 2035.
North America will remain the largest market, accounting for ~43% of revenues in 2025 (USD 26.7 billion), supported by advanced infrastructure, FDA oversight, and high R&D spending.
Asia-Pacific will see the fastest growth, driven by expanding trial ecosystems in India and China, CRO partnerships, and government-backed initiatives.
Europe will sustain growth with its strong biologics pipeline, harmonized regulatory environment, and emphasis on sustainability.
By 2035, clinical trial services will be anchored in patient-centric, digitally optimized ecosystems, ensuring faster, more efficient, and sustainable execution worldwide.
To know more about the Clinical Trial Services Market - Download our Sample Report: https://marketgenics.co/download-report-sample/clinical-trial-services-market-42377
Key Drivers, Challenges, and Opportunities
Driver - Patient-Centric Protocol Execution with Digital Tools
The shift toward decentralized and hybrid trials is driving demand for integrated solutions blending eConsent, remote monitoring, wearables, and curated site networks.
Thermo Fisher Scientific's CorEvitas registries and home health integrations exemplify this trend, enabling adaptive designs and improving retention.
Restraint - Data Integrity and Interoperability Barriers
The rise of multimodal studies with imaging, biomarkers, and eCOA data introduces integration challenges. Workflow interoperability between platforms remains limited, creating backlogs and quality-control issues.
Despite AI-enabled quality checks, providers face ongoing burdens reconciling heterogeneous datasets across global sites.
Opportunity - Real-World Evidence and AI-Pathology Expansion
Growing use of real-world evidence (RWE) and AI-driven computational pathology creates new service lines. Labcorp's partnership with PathAI in 2025 enables algorithm-derived oncology endpoints, while Thermo Fisher's therapeutic registries provide longitudinal data for external control arms.
Together, these advances open premium analytics-infused service opportunities for CROs.
Key Trend - Sustainability and Site-Centric Execution
Sustainability is emerging as a defining trend in trial services, with providers embedding green logistics, optimized site visits, and digital monitoring into operations.
In June 2025, Thermo Fisher and the Society for Clinical Research Sites (SCRS) launched a sustainability advocacy group, standardizing eco-efficient practices that also enhance retention and predictability.
Medpace's Cincinnati campus redevelopment, including a new pharmacology unit, highlights the growing emphasis on concentrated, high-throughput infrastructure designed for both efficiency and sustainability.
This "responsibility-by-design" model is rapidly becoming a competitive differentiator in global trial execution.
Buy Now: https://marketgenics.co/buy/clinical-trial-services-market-42377
Segmental Insights
Clinical Trial Management & Monitoring Leads (~32% share in 2025)
This segment dominates due to rising protocol complexity, decentralized trial adoption, and stringent oversight requirements. ICON plc expanded its AI-based monitoring platform in 2025, reducing on-site visits while ensuring compliance with ICH E6(R3) guidelines.
Laboratory and Data Management Services Accelerating
The growth of precision medicine is boosting demand for lab testing, NGS, and AI-based data analytics, with providers like Labcorp and Parexel enhancing platforms to support oncology and rare disease trials.
Regional Highlights
North America:
Leads with USD 26.7 billion revenue in 2025, supported by advanced infrastructure, large sponsor presence, and strong adoption of AI-enabled designs. Example: Pfizer's partnership with Medidata in 2025 integrated advanced analytics to reduce trial cycle times.
Asia-Pacific:
Fastest-growing region, with India and China serving as trial hubs due to large patient pools, favorable government policies, and CRO expansions.
Europe:
Strong biologics and rare disease focus, supported by EMA harmonization and green trial infrastructure.
Competitive Landscape
The market is moderately consolidated, with Tier-1 CROs holding ~35% share in 2025.
Tier 1 Leaders
IQVIA
Labcorp Drug Development
PPD (Thermo Fisher)
Parexel
ICON plc
Tier 2 Players
Syneos Health, Charles River, WuXi AppTec, Medpace
Tier 3 Players
Clinipace, Novotech, Veristat, and regional niche providers
Competition is intensifying as CROs integrate AI, RWE, and sustainability frameworks to differentiate services and deepen sponsor partnerships.
Get a preview of our Clinical Trial Services Market Playbook - your guide to GTM strategy, competitive intelligence, supplier dynamics, and Consumer Behavior Analysis: https://marketgenics.co/playbook/clinical-trial-services-market-42377
Future Outlook
By 2035, clinical trial services will evolve into a digitally integrated, sustainability-anchored ecosystem, where AI, real-world evidence, and decentralized trial models converge.
Key growth areas will include:
AI-enhanced trial design and monitoring
RWE-driven recruitment and analytics
Patient-centric, decentralized execution models
Green and site-optimized operations
These shifts will position clinical trial services as a strategic enabler of faster, more efficient, and equitable global drug development.
Prominent Companies Operating in the Global Clinical Trial Services Market:
IQVIA, Labcorp Drug Development, PPD (Thermo Fisher Scientific), Parexel, ICON plc, Syneos Health, Charles River, WuXi AppTec, Medpace, Clinipace, Novotech, Veristat, and others.
About Us
MarketGenics is a global market research and management consulting company empowering decision makers across healthcare, technology, and policy domains. Our mission is to deliver granular market intelligence combined with strategic foresight to accelerate sustainable growth.
We support clients across strategy development, product innovation, healthcare infrastructure, and digital transformation.
Contact:
Mr. Debashish Roy
MarketGenics India Pvt. Ltd.
800 N King Street, Suite 304 #4208, Wilmington, DE 19801, United States
USA: +1 (302) 303-2617
Email: sales@marketgenics.co
Website: https://marketgenics.co
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Clinical Trial Services Market to Reach USD 135 Billion by 2035, Driven by AI, Decentralized Trials, and Sustainability-Centered Operations here
News-ID: 4205218 • Views: …
More Releases from MarketGenics India Pvt. Ltd.
APAC Deepfake Detection Market Accelerates as Governments Tighten Digital Trust …
A Market Transforming How the World Verifies Reality
The global deepfake detection technology market, valued at USD 0.6 billion in 2025, is positioned to accelerate at a powerful 37.2% CAGR, reaching USD 15.1 billion by 2035.
This growth is driven by one undeniable truth:
Synthetic media is reshaping the threat landscape faster than humans can recognize it.
Deepfake detection technologies now determine:
How newsrooms verify breaking content
How financial institutions prevent identity-spoofing
How governments protect election integrity
How…
Travel & Expense Management Software Market Signals a Digital Pivot | AI, Cloud …
The Travel and Expense Management (TEM) Market Crossroads | A Sector Accelerating, Repricing Efficiency, and Redrawing the Corporate Spend Map
(Is TEM a Back-Office Tool-or the Operating System of the Next Enterprise Economy?)
For years, the travel and expense management software market lived in the administrative shadows-handed off to finance teams, constrained by spreadsheets, and dismissed as a routine cost-control tool. But the numbers now tell a radically different story.
In 2025, the…
Oilfield Equipment Market hits USD 116.2B in 2025 and grows to USD 156.5B by 203 …
Oilfield Equipment Market | The $156.5B Hardware Backbone of the Global Energy System
Every headline loves clean energy. Yet the global energy mix still demands a brutal truth: oil and gas remain the world's primary supply of heat, mobility, and petrochemicals - and the machines that drill, lift, complete, and produce hydrocarbons continue to define industrial capability.
That's why the Oilfield Equipment Market remains a strategic industry - not a relic.
In 2025,…

Machine Tools Market 2025-2035 | USD 109.9B Growth, CNC & Automation Trends
Machine Tools Market | The $109.9B Intelligence Engine of Global Manufacturing
Factories don't work without machine tools. They shape, cut, drill, grind, and define the physical world around us. Yet most end-products - cars, aircraft parts, electronics housings, surgical devices - never reveal the precision machinery behind them.
The Machine Tools Market is the invisible infrastructure that turns digital models into physical reality.
In 2025, the global Machine Tools Market stands at USD…
More Releases for Trial
Clinical Trial Investigative Site Network Market Clinical Trial Investigative Si …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trial Investigative Site Network Market - (By Therapeutic Areas (Oncology, Cardiology, CNS, Pain Management, Endocrine, Others), By Phase (Phase I, Phase II, Phase III, Phase IV), By End-use (Sponsor, CRO)), Trends, Industry Competition Analysis, Revenue and Forecast To 2034."
According to the latest research by InsightAce Analytic, the Global Clinical Trial Investigative Site Network Market…
Transformative Trends Impacting the Electronic Trial Master File (eTMF) Systems …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Electronic Trial Master File (eTMF) Systems Market Size By 2025?
The market size of the electronic trial master file (eTMF) systems has experienced fast growth over recent years. The market is projected to increase from $1.36 billion in 2024 to $1.55 billion in 2025, with…
Transformative Trends Impacting the Electronic Trial Master File (eTMF) Systems …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Electronic Trial Master File (eTMF) Systems Market Size By 2025?
The market size of the electronic trial master file (eTMF) systems has experienced fast growth over recent years. The market is projected to increase from $1.36 billion in 2024 to $1.55 billion in 2025, with…
Clinical Trial Imaging market
The Clinical Trial Imaging market crossed the US$ 1.09 billion mark in 2022 and is expected to hit US$ 1.94 billion by 2030, recording a CAGR of 7.5% during the forecast period.
Rising R&D spending, a rapidly growing pharmaceutical industry, and an increase in the number of contract research organizations are some of the major factors driving the market's growth. There has been an increase in pharmaceutical companies due to the…
Clinical Trial Logistics
Clinical Trial Logistics
16th to 17th May 2011, Marriott Regents Park, London, United Kingdom.
It currently costs just over £500 million ($800 million) to bring a new chemical to market and development timelines continue to fall in the 10-15 year range. A key reason for high R&D costs is due to logistical failures including failure to recruit patients on time. A way to avoid this is to move clinical trials…
Clinical Trial Logistics
Announcing SMi's 5th annual…
Clinical Trial Logistics conference
16th and 17th May 2011, Central London, UK
www.smi-online.co.uk/2011logistics-london6.asp
It currently costs just over £500 million ($800 million) to bring a new chemical to market and development timelines continue to fall in the 10-15 year range. A key reason for high R&D costs is due to logistical failures including failure to recruit patients on time. A way to avoid this is to move clinical…