Press release
Clinical Trial Supplies Market to Reach USD 9.7 Billion by 2035, Driven by Biologics, Decentralized Trials, and AI-Powered Supply Chains
Clinical Trial Supplies Market to Reach USD 9.7 Billion by 2035, Driven by Biologics, Decentralized Trials, and AI-Powered Supply
MarketGenics, a leading research firm, highlights clinical trial supplies as a critical backbone of global drug development, enabling precision logistics, compliance, and patient-centered trial participation in an era of accelerated therapeutic innovation.
Get the Detailed Industry Analysis (including the Table of Contents, List of Figures, and List of Tables) - from the Clinical Trial Supplies Market Research Report: https://marketgenics.co/press-releases/clinical-trial-supplies-market-01651
Recent Developments Shaping the Market
DHL Scales Healthcare Logistics Infrastructure
In May 2025, DHL Group announced a €3.5 billion multiyear capex plan and a new €300 million cost-reduction program, alongside the opening of a 32,000 m2 certified pharma hub near Frankfurt to expand controlled-temperature warehousing and trial supply services.
UPS Expands Cold-Chain Capabilities
In April 2025, UPS acquired Andlauer Healthcare Group for USD 1.6 billion, strengthening its global temperature-controlled logistics and cryogenic handling solutions, especially for biologics and cell therapies.
Catalent Advances Decentralized Supply Models
In January 2024, Catalent launched a direct-to-patient delivery and telehealth-friendly packaging solution, addressing growing demand for decentralized and hybrid trial models that improve patient retention.
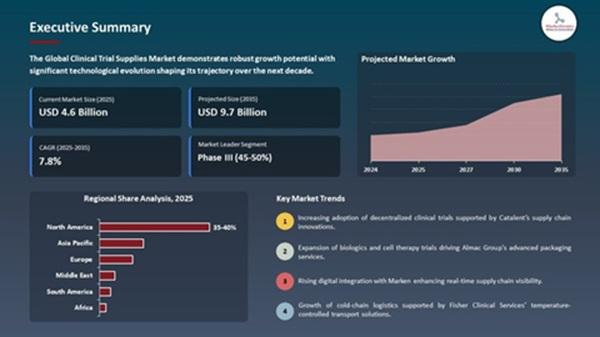
Clinical Trial Supplies Market Forecast 2035
The market is set to create an incremental opportunity of USD 5.1 billion between 2025 and 2035.
North America will remain the largest market, supported by advanced trial infrastructure, FDA-regulated compliance, and a strong pipeline of oncology and cell therapy trials.
Asia-Pacific will record the fastest growth, driven by government-backed trial ecosystems in India and China and the increasing presence of global CROs.
Europe will sustain steady expansion with strong biologics R&D and a robust cold-chain network.
By 2035, AI-enabled, patient-centric supply chains will define global trial logistics, minimizing delays, ensuring compliance, and improving trial efficiency.
To know more about the Clinical Trial Supplies Market - Download our Sample Report: https://marketgenics.co/download-report-sample/clinical-trial-supplies-market-01651
Key Drivers, Challenges, and Opportunities
Driver - Complexity of Biologics and Personalized Medicine
The surge in biologics, cell, and gene therapies is reshaping supply needs. Lonza and Marken have expanded cryogenic storage and ultra-low temperature logistics to support these next-generation therapies.
Restraint - High Costs in Cold-Chain and Cross-Border Compliance
Advanced cryogenic handling systems remain cost-intensive, creating barriers for small- and mid-size biotech firms. Marken noted the heavy financial burden of maintaining global CAR-T logistics despite surging demand.
Opportunity - Growth of Decentralized Clinical Trials (DCTs)
Direct-to-patient supply chains, integrated inventory tracking, and telemedicine-enabled packaging are transforming participation models. These innovations open opportunities for providers who can deliver patient-specific supply models at scale.
Key Trend - AI and Digitalization in Trial Supply Chains
AI-driven supply chain management is emerging as a game-changer.
In March 2024, Parexel and Microsoft Azure introduced AI-based predictive analytics for oncology trials, enabling precise forecasting of supply demand and real-time risk mitigation.
Digital platforms, blockchain-based compliance tools, and smart packaging are reducing inventory wastage, trial delays, and regulatory risks, making AI a cornerstone of future trial supply strategies.
Buy Now: https://marketgenics.co/buy/clinical-trial-supplies-market-01651
Segmental Insights
Phase III Trials Drive Peak Demand (~47% share in 2025)
Phase III trials involve the largest patient populations, longest durations, and most complex multi-country logistics, fueling demand for high-volume packaging, temperature-controlled shipping, and compliance monitoring.
Decentralized and Hybrid Trials Fuel New Models
Suppliers are increasingly offering direct-to-patient (DTP) and home delivery solutions, with adaptive trial designs requiring flexible supply systems that can scale dynamically across regions.
Regional Highlights
North America:
Leads with ~59.7% share (USD 2.7 billion in 2025), powered by strong R&D infrastructure, FDA-regulated compliance frameworks, and large volumes of oncology and rare-disease trials.
Example: Thermo Fisher's 2024 facility expansion in Kentucky to manage complex multi-country biologics trials.
Asia-Pacific:
Fastest-growing region, supported by rapid patient recruitment in India and China, CRO expansion, and increasing clinical trial outsourcing.
Europe:
Growth led by biologics cold-chain investments and strong regulatory frameworks (EMA compliance).
Competitive Landscape
The market is moderately consolidated, with Tier-1 leaders holding ~45% share in 2025.
Tier 1 Leaders
Thermo Fisher Scientific (Fisher Clinical Services)
Catalent
PCI Pharma Services
Almac Group
UPS/Marken
Lonza
Parexel
Tier 2 Players
Clinigen, Eurofins, Vetter Pharma, Cambrex, Movianto
Tier 3 Players
Biocair, KLIFO, Sentry BioPharma
Competition is intensifying as providers integrate cold-chain, AI-enabled tracking, and patient-centric models to gain market leadership.
Get a preview of our Clinical Trial Supplies Market Playbook - your guide to GTM strategy, competitive intelligence, supplier dynamics, and Consumer Behavior Analysis: https://marketgenics.co/playbook/clinical-trial-supplies-market-01651
Future Outlook
By 2035, the clinical trial supplies market will transform into a digitally driven, patient-centered ecosystem, where real-time analytics, sustainable packaging, and decentralized distribution models define the industry.
Key growth areas include:
AI-based demand forecasting and compliance management
Smart packaging and blockchain for real-time transparency
Cryogenic logistics for cell and gene therapies
Patient-centric DTP and home-delivery trial supply systems
This evolution will position the clinical trial supplies sector as a strategic enabler of global drug development, ensuring speed, compliance, and equity in life sciences innovation.
Prominent Companies Operating in the Global Clinical Trial Supplies Market:
Thermo Fisher Scientific, Catalent, PCI Pharma Services, Almac Group, UPS/Marken, Lonza, Parexel, DHL Group, Clinigen, Eurofins, Vetter Pharma, Cambrex, Movianto, Biocair, KLIFO, Sentry BioPharma.
About Us
MarketGenics is a global market research and management consulting company empowering decision makers across healthcare, technology, and policy domains. Our mission is to deliver granular market intelligence combined with strategic foresight to accelerate sustainable growth.
We support clients across strategy development, product innovation, healthcare infrastructure, and digital transformation.
Contact:
Mr. Debashish Roy
MarketGenics India Pvt. Ltd.
800 N King Street, Suite 304 #4208, Wilmington, DE 19801, United States
USA: +1 (302) 303-2617
Email: sales@marketgenics.co
Website: https://marketgenics.co
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Clinical Trial Supplies Market to Reach USD 9.7 Billion by 2035, Driven by Biologics, Decentralized Trials, and AI-Powered Supply Chains here
News-ID: 4205207 • Views: …
More Releases from MarketGenics India Pvt. Ltd.
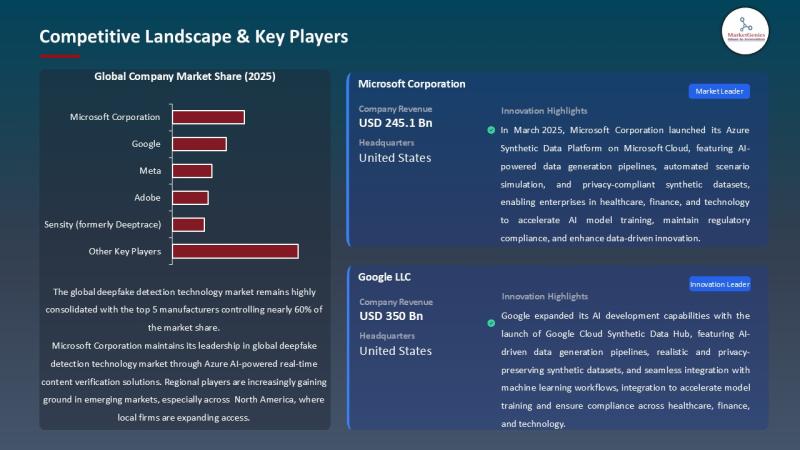
APAC Deepfake Detection Market Accelerates as Governments Tighten Digital Trust …
A Market Transforming How the World Verifies Reality
The global deepfake detection technology market, valued at USD 0.6 billion in 2025, is positioned to accelerate at a powerful 37.2% CAGR, reaching USD 15.1 billion by 2035.
This growth is driven by one undeniable truth:
Synthetic media is reshaping the threat landscape faster than humans can recognize it.
Deepfake detection technologies now determine:
How newsrooms verify breaking content
How financial institutions prevent identity-spoofing
How governments protect election integrity
How…
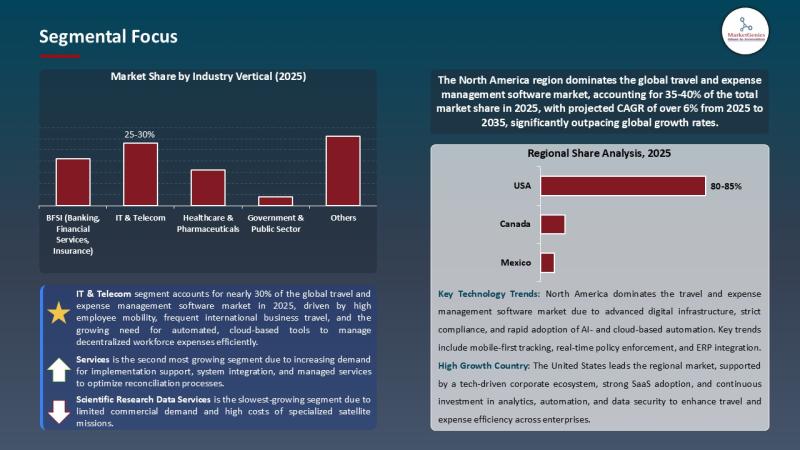
Travel & Expense Management Software Market Signals a Digital Pivot | AI, Cloud …
The Travel and Expense Management (TEM) Market Crossroads | A Sector Accelerating, Repricing Efficiency, and Redrawing the Corporate Spend Map
(Is TEM a Back-Office Tool-or the Operating System of the Next Enterprise Economy?)
For years, the travel and expense management software market lived in the administrative shadows-handed off to finance teams, constrained by spreadsheets, and dismissed as a routine cost-control tool. But the numbers now tell a radically different story.
In 2025, the…
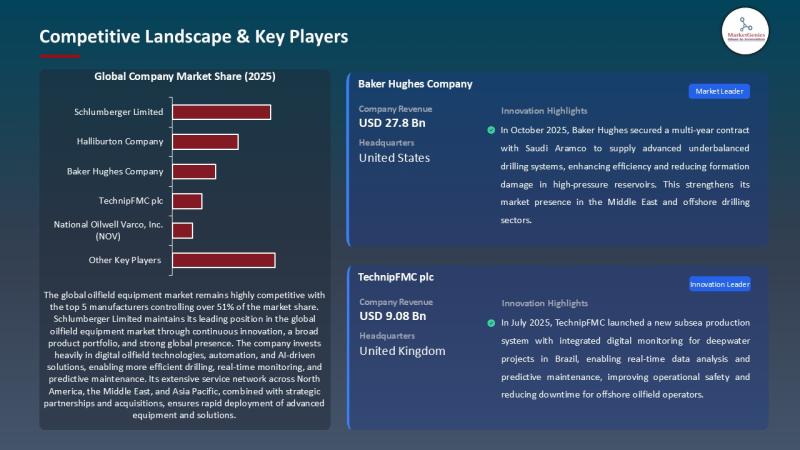
Oilfield Equipment Market hits USD 116.2B in 2025 and grows to USD 156.5B by 203 …
Oilfield Equipment Market | The $156.5B Hardware Backbone of the Global Energy System
Every headline loves clean energy. Yet the global energy mix still demands a brutal truth: oil and gas remain the world's primary supply of heat, mobility, and petrochemicals - and the machines that drill, lift, complete, and produce hydrocarbons continue to define industrial capability.
That's why the Oilfield Equipment Market remains a strategic industry - not a relic.
In 2025,…

Machine Tools Market 2025-2035 | USD 109.9B Growth, CNC & Automation Trends
Machine Tools Market | The $109.9B Intelligence Engine of Global Manufacturing
Factories don't work without machine tools. They shape, cut, drill, grind, and define the physical world around us. Yet most end-products - cars, aircraft parts, electronics housings, surgical devices - never reveal the precision machinery behind them.
The Machine Tools Market is the invisible infrastructure that turns digital models into physical reality.
In 2025, the global Machine Tools Market stands at USD…
More Releases for Trial
Clinical Trial Investigative Site Network Market Clinical Trial Investigative Si …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trial Investigative Site Network Market - (By Therapeutic Areas (Oncology, Cardiology, CNS, Pain Management, Endocrine, Others), By Phase (Phase I, Phase II, Phase III, Phase IV), By End-use (Sponsor, CRO)), Trends, Industry Competition Analysis, Revenue and Forecast To 2034."
According to the latest research by InsightAce Analytic, the Global Clinical Trial Investigative Site Network Market…
Transformative Trends Impacting the Electronic Trial Master File (eTMF) Systems …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Electronic Trial Master File (eTMF) Systems Market Size By 2025?
The market size of the electronic trial master file (eTMF) systems has experienced fast growth over recent years. The market is projected to increase from $1.36 billion in 2024 to $1.55 billion in 2025, with…
Transformative Trends Impacting the Electronic Trial Master File (eTMF) Systems …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Electronic Trial Master File (eTMF) Systems Market Size By 2025?
The market size of the electronic trial master file (eTMF) systems has experienced fast growth over recent years. The market is projected to increase from $1.36 billion in 2024 to $1.55 billion in 2025, with…
Clinical Trial Imaging market
The Clinical Trial Imaging market crossed the US$ 1.09 billion mark in 2022 and is expected to hit US$ 1.94 billion by 2030, recording a CAGR of 7.5% during the forecast period.
Rising R&D spending, a rapidly growing pharmaceutical industry, and an increase in the number of contract research organizations are some of the major factors driving the market's growth. There has been an increase in pharmaceutical companies due to the…
Clinical Trial Logistics
Clinical Trial Logistics
16th to 17th May 2011, Marriott Regents Park, London, United Kingdom.
It currently costs just over £500 million ($800 million) to bring a new chemical to market and development timelines continue to fall in the 10-15 year range. A key reason for high R&D costs is due to logistical failures including failure to recruit patients on time. A way to avoid this is to move clinical trials…
Clinical Trial Logistics
Announcing SMi's 5th annual…
Clinical Trial Logistics conference
16th and 17th May 2011, Central London, UK
www.smi-online.co.uk/2011logistics-london6.asp
It currently costs just over £500 million ($800 million) to bring a new chemical to market and development timelines continue to fall in the 10-15 year range. A key reason for high R&D costs is due to logistical failures including failure to recruit patients on time. A way to avoid this is to move clinical…