Press release
Metachromatic Leukodystrophy Pipeline 2025: Pioneering Clinical Developments by 6+ Global Leaders - DelveInsight | Featuring Takeda, Denali Therapeutics, Orchard Therapeutics, Homology Medicines, Pass
DelveInsight's, "Metachromatic Leukodystrophy - Pipeline Insight, 2025," report provides comprehensive insights about 6+ companies and 6+ pipeline drugs in Metachromatic Leukodystrophy pipeline landscape. It covers the pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.With Metachromatic Leukodystrophy (MLD) increasingly affecting populations worldwide and contributing to comorbidities such as diabetes, cardiovascular disease, and certain cancers, there is a rising demand for safer and more effective treatment options. DelveInsight reports that the MLD pipeline includes over six pharmaceutical and biotech companies actively developing more than six therapeutic candidates for the condition. These therapies are at various stages of clinical and preclinical development, reflecting significant innovation and commitment to tackling this critical public health challenge.
DelveInsight's "Metachromatic Leukodystrophy Pipeline Insight 2025" offers an in-depth strategic analysis of the current R&D landscape. It examines clinical trial progress, emerging therapies, mechanisms of action, competitive positioning, and key company initiatives. The report serves as an essential resource for stakeholders-including researchers, healthcare investors, and decision-makers-seeking insights into the evolving MLD therapeutics market and the advancements shaping its future.
Explore the Cutting-Edge Landscape of Metachromatic Leukodystrophy Drug Development [https://www.delveinsight.com/report-store/metachromatic-leukodystrophy-mld-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
Key Takeaways from the Metachromatic Leukodystrophy Pipeline Report
*
DelveInsight's Metachromatic Leukodystrophy (MLD) pipeline report highlights a dynamic landscape with over six active companies developing more than six therapeutic candidates for MLD treatment.
*
In March 2024, the U.S. Food and Drug Administration (FDA) approved Lenmeldy (atidarsagene autotemcel), marking the first gene therapy authorized in the U.S. for pediatric patients with MLD. Developed by Orchard Therapeutics, Lenmeldy targets children with pre-symptomatic late infantile, pre-symptomatic early juvenile, or early symptomatic early juvenile forms of the disease.
*
Key players in the MLD space, including Takeda, Denali Therapeutics, Orchard Therapeutics, Homology Medicines, Passage Bio, ArmaGen Technologies, and others, are actively evaluating new therapies to enhance the treatment landscape.
*
Promising MLD pipeline candidates currently in various stages of development include OTL-200, TAK-611, and several others.
Metachromatic Leukodystrophy Overview:
Metachromatic leukodystrophy (MLD) is a rare genetic disorder characterized by the accumulation of sulfatides, a type of fat, which causes the degradation of the myelin sheath-the protective layer surrounding nerves-in both the central and peripheral nervous systems. The disease is categorized into three forms according to the age at onset: late-infantile, juvenile, and adult.
MLD is most commonly caused by mutations in the ASA (or ARSA) gene, with occasional cases linked to PSAP gene mutations. Diagnosis is usually prompted by a distinctive pattern of progressive neurological decline. In the late-infantile form, early signs often include walking difficulties, such as toe walking or foot drop. Adult-onset MLD may first appear as speech difficulties, behavioral changes, or academic challenges, while juvenile MLD can present with either motor or cognitive impairments.
For children who are pre-symptomatic or exhibit mild symptoms, stem cell transplantation may be considered. In most other cases, treatment remains supportive, aiming to manage symptoms and preserve quality of life.
Download the Metachromatic Leukodystrophy sample report to know in detail about the Metachromatic Leukodystrophy treatment market [https://www.delveinsight.com/sample-request/metachromatic-leukodystrophy-mld-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
Metachromatic Leukodystrophy Pipeline Analysis
The Metachromatic Leukodystrophy pipeline insights report 2025, provides insights into:
*
Provides comprehensive insights into key companies developing therapies in the Metachromatic Leukodystrophy Market.
*
Categorizes Metachromatic Leukodystrophy therapeutic companies by development stage: early, mid, and late-stage.
*
Highlights major companies involved in targeted therapy development, including both active and inactive (paused/discontinued) projects.
*
Reviews emerging Metachromatic Leukodystrophy drugs under development based on:
*
Stage of development
*
Metachromatic Leukodystrophy Route of administration
*
Target receptor
*
Monotherapy vs. combination therapy
*
Metachromatic Leukodystrophy Mechanism of action
*
Molecular type
*
Offers detailed analysis of:
*
Company-to-company and company-academia collaborations
*
Metachromatic Leukodystrophy Licensing agreements
*
Funding and investment activities supporting future Metachromatic Leukodystrophy market advancement.
Unlock key insights into emerging Metachromatic Leukodystrophy therapies and market strategies here: https://www.delveinsight.com/report-store/metachromatic-leukodystrophy-mld-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr
Metachromatic Leukodystrophy Emerging Drugs
*
OTL-200: Orchard Therapeutics
OTL-200, known as Libmeldy in the European Union, is an ex vivo autologous gene therapy that utilizes CD34+ cells enriched with hematopoietic stem and progenitor cells (HSPCs). These cells are genetically engineered outside the body with a lentiviral vector to deliver the human arylsulfatase-A (ARSA) gene. The therapy received approval from the European Medicines Agency (EMA) in 2020. However, OTL-200 remains investigational and has not yet been authorized by the U.S. Food and Drug Administration (FDA) or other regulatory agencies worldwide.
*
TAK-611: Takeda
TAK-611, a recombinant version of cerebroside sulfatase, is being developed by Takeda as an enzyme replacement therapy for metachromatic leukodystrophy (MLD), a rare genetic disorder characterized by the accumulation of toxic lipids that harm the myelin sheath surrounding nerve cells. The therapy is currently undergoing Phase II clinical trials for MLD treatment.
Metachromatic Leukodystrophy Pipeline Therapeutic Assessment
Metachromatic Leukodystrophy Assessment by Product Type
- Mono
- Combination
- Mono/Combination
Metachromatic Leukodystrophy By Stage
- Late-stage products (Phase III)
- Mid-stage products (Phase II)
- Early-stage product (Phase I) along with the details of
- Pre-clinical and Discovery stage candidates
- Discontinued & Inactive candidates
Metachromatic Leukodystrophy Assessment by Route of Administration
- Oral
- Parenteral
- Intravenous
- Subcutaneous
- Topical
Metachromatic Leukodystrophy Assessment by Molecule Type
- Recombinant fusion proteins
- Small molecule
- Monoclonal antibody
- Peptide
- Polymer
- Gene therapy
Download sample pages to get an in-depth assessment of the emerging Metachromatic Leukodystrophy therapies and key Metachromatic Leukodystrophy companies [https://www.delveinsight.com/sample-request/metachromatic-leukodystrophy-mld-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
Table of Contents
1. Report Introduction
2. Executive Summary
3. Metachromatic Leukodystrophy Current Treatment Patterns
4. Metachromatic Leukodystrophy - DelveInsight's Analytical Perspective
5. Therapeutic Assessment
6. Metachromatic Leukodystrophy Late-Stage Products (Phase-III)
7. Metachromatic Leukodystrophy Mid-Stage Products (Phase-II)
8. Early Stage Products (Phase-I)
9. Pre-clinical Products and Discovery Stage Products
10. Inactive Products
11. Dormant Products
12. Metachromatic Leukodystrophy Discontinued Products
13. Metachromatic Leukodystrophy Product Profiles
14. Metachromatic Leukodystrophy Key Companies
15. Metachromatic Leukodystrophy Key Products
16. Dormant and Discontinued Products
17. Metachromatic Leukodystrophy Unmet Needs
18. Metachromatic Leukodystrophy Future Perspectives
19. Metachromatic Leukodystrophy Analyst Review
20. Appendix
21. Report Methodology
Request the sample PDF to get detailed insights about the Metachromatic Leukodystrophy pipeline reports offerings [https://www.delveinsight.com/report-store/metachromatic-leukodystrophy-mld-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports Pharma companies by providing comprehensive end-to-end solutions to improve their performance.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Kritika Rehani
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=metachromatic-leukodystrophy-pipeline-2025-pioneering-clinical-developments-by-6-global-leaders-delveinsight-featuring-takeda-denali-therapeutics-orchard-therapeutics-homology-medicines-pass]
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: Nevada
Country: United States
Website: https://www.delveinsight.com/
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Metachromatic Leukodystrophy Pipeline 2025: Pioneering Clinical Developments by 6+ Global Leaders - DelveInsight | Featuring Takeda, Denali Therapeutics, Orchard Therapeutics, Homology Medicines, Pass here
News-ID: 4198417 • Views: …
More Releases from ABNewswire
OTC Micro-Caps: AMLM, RNWF, BMXI, RECX, VMHG Gain Momentum in February 2026
As investors screen for "OTC stocks to watch 2026," "critical minerals stocks," "micro-cap gold spinoffs," and "fusion energy plays," several emerging OTC names are positioning themselves around identifiable catalysts spanning mining, advanced energy, specialty manufacturing, and marine yacht brokerage.
American Lithium Minerals (OTCID: AMLM) continues to reposition as a diversified, U.S.-anchored critical minerals company. The Company expanded its Sarcobatus Lithium Property in Nye County, Nevada, the project sits within a key…
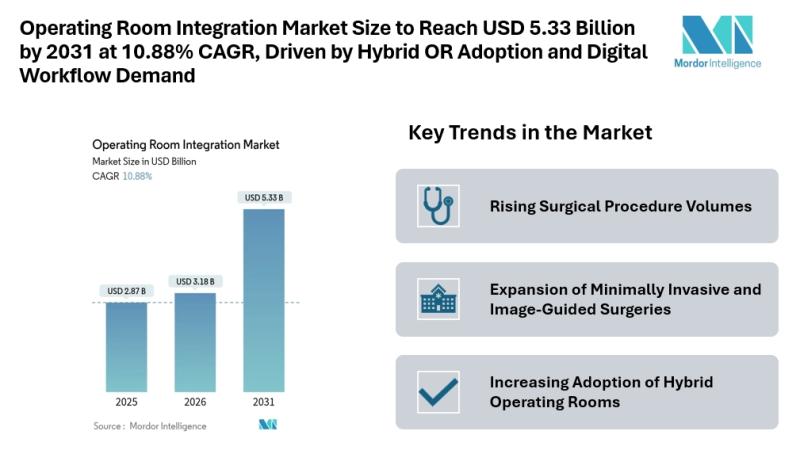
Operating Room Integration Market Size to Reach USD 5.33 Billion by 2031 at 10.8 …
Mordor Intelligence has published a new report on the operating room integration market, offering a comprehensive analysis of trends, growth drivers, and future projections.
Operating Room Integration Market Analysis
According to Mordor Intelligence, the operating room integration market size [https://www.mordorintelligence.com/industry-reports/operating-room-integration?utm_source=abnewswire] is projected to USD 3.18 billion in 2026 and reach USD 5.33 billion by 2031, registering a CAGR of 10.88% during the forecast period. The operating room integration market growth reflects the…
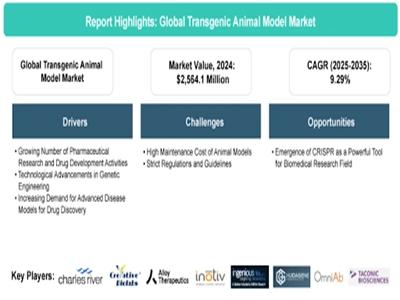
Transgenic Animal Model Market Forecast 2025-2035: Growth, Trends, and Future Ou …
Transgenic animal models play a critical role in oncology, immunology, rare disease research, and biologics development by enabling highly accurate preclinical testing. Growth in cell and gene therapies, expanding pharmaceutical R&D pipelines, and increased public-private research collaborations are further accelerating adoption.
According to BIS Research, the global [https://bisresearch.com/industry-report/transgenic-animal-model-market.html?utm_source=Monika&utm_medium=Abnewswire&utm_campaign=monika_pr]is projected to reach $6,799.3 million by 2035, growing at a compound annual growth rate (CAGR) of 9.29% during the forecast period 2025-2035. The…

Cheapest ZZ Top Tickets 2026: Dos Amigos Tour Dates - Promo CITY10 CapitalCityTi …
ZZ Top teams up with Dwight Yoakam for the epic Dos Amigos Tour in 2026! Spring run starts March 26 at Dacotah Bank Center (Brookings, SD), hits INTRUST Bank Arena (Wichita, KS, Mar 28), Simmons Bank Arena (North Little Rock, AR, Apr 17), Orion Amphitheater (Huntsville, AL, Apr 18), Azura Amphitheater (Bonner Springs, KS, Apr 25), Lauridsen Amphitheater (Des Moines, IA, May 7), Koka Booth Amphitheatre (Cary, NC, May 22),…
More Releases for Metachromatic
Metachromatic Leukodystrophy Market to Reach USD 182.6 Million by 2034
Pune, India - December 2025 - The global Metachromatic Leukodystrophy (MLD) Market, valued at USD 62.4 million in 2024, is projected to reach USD 182.6 million by 2034, growing at a powerful 11.2% CAGR (2025-2034), according to Exactitude Consultancy. Rapid advancements in gene therapy, improved diagnostic capabilities, and growing awareness of rare neurodegenerative disorders are accelerating market growth.
Download Full PDF Sample Copy of Market Report
https://exactitudeconsultancy.com/request-sample/72057
Market Summary
The Metachromatic Leukodystrophy Market…
Metachromatic Leukodystrophy (MLD) market is expected to reach USD 1.1 billion b …
Metachromatic Leukodystrophy (MLD) is a rare, inherited lysosomal storage disorder caused by a deficiency in the enzyme arylsulfatase A (ARSA), leading to toxic accumulation of sulfatides in the nervous system. This results in progressive demyelination, neurodegeneration, and severe physical and cognitive decline. MLD manifests in late-infantile, juvenile, and adult-onset forms, with the late-infantile subtype being the most aggressive and fatal without intervention.
Download Full PDF Sample Copy of Market Report @…
Metachromatic Leukodystrophy Treatment Market to Exceed USD 479.51 Million by 20 …
Metachromatic leukodystrophy (MLD) is a rare but devastating genetic disorder that primarily affects children and leads to progressive deterioration of the nervous system. Historically, the absence of effective treatment options and the rapid progression of the disease left families with few avenues for intervention. However, recent breakthroughs in gene therapy, enzyme replacement therapy (ERT), and early diagnostic screening have changed the landscape dramatically.
Download Full PDF Sample Copy of Market Report…
Metachromatic Leukodystrophy Pipeline Insight 2025: Advancing Gene Therapies and …
DelveInsight's "Metachromatic Leukodystrophy - Pipeline Insight, 2025" report delivers a detailed overview of the therapeutic development landscape targeting this rare, inherited lysosomal storage disorder caused by arylsulfatase A (ARSA) deficiency. Characterized by progressive demyelination affecting the central and peripheral nervous systems, Metachromatic Leukodystrophy (MLD) often leads to severe motor and cognitive decline, with limited treatment options available until recently.
In 2025, the MLD pipeline is dominated by advanced gene therapies and…
Metachromatic Leukodystrophy Treatment Market is expected to show growth from 20 …
Metachromatic Leukodystrophy Treatment Market was valued at USD 3.53 Bn. in 2023 and is expected to reach USD 7.06 Bn. by 2030, at a CAGR of 10.4% over the forecast period (2024-2030).
Metachromatic Leukodystrophy Treatment Market Overview
Metachromatic leukodystrophy (MLD) is a rare genetic disorder caused by a deficiency of the enzyme arylsulfatase A, which is vital for maintaining myelin, the protective layer around nerve fibers. Without this enzyme, myelin deteriorates,…
Metachromatic Leukodystrophy Pipeline, FDA Approvals, Clinical Trials Developmen …
DelveInsight's, "Metachromatic Leukodystrophy Pipeline Insight 2024" report provides comprehensive insights about 6+ companies and 6+ pipeline drugs in the Metachromatic Leukodystrophy pipeline landscape. It covers the Metachromatic Leukodystrophy pipeline drug profiles, including clinical and nonclinical stage products. It also covers the Metachromatic Leukodystrophy therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Key Takeaways from the Metachromatic Leukodystrophy…
