Press release
Axillary hyperhidrosis Pipeline 2025: Pioneering Clinical Developments by 3+ Global Leaders - DelveInsight | Highlighting GlaxoSmithKline, Medytox, Theravidam, Candesant Biomedical
DelveInsight's, "Axillary Hyperhidrosis - Pipeline Insight, 2025," report provides comprehensive insights about 3+ companies and 3+ pipeline drugs in Axillary Hyperhidrosis pipeline landscape. It covers the pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.With Axillary hyperhidrosis becoming increasingly prevalent worldwide and contributing to comorbidities such as diabetes, cardiovascular disease, and certain cancers, the need for safer and more effective treatments is rising. DelveInsight reports that the Axillary hyperhidrosis pipeline includes 3+ pharmaceutical and biotech companies actively developing 3+ therapeutic candidates for this condition. These therapies are in various stages of clinical and preclinical development, reflecting strong innovation and a commitment to tackling a significant public health challenge.
DelveInsight's "Axillary Hyperhidrosis Pipeline Insight 2025" provides a comprehensive analysis of the current R&D landscape, including clinical trial progress, emerging therapies, mechanisms of action, competitive positioning, and key company initiatives. The report is an essential resource for stakeholders-such as researchers, healthcare investors, and decision-makers-seeking insights into the evolving Axillary hyperhidrosis therapeutics market and the breakthroughs shaping its future.
Explore the Cutting-Edge Landscape of Axillary hyperhidrosis Drug Development [https://www.delveinsight.com/report-store/axillary-hyperhidrosis-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
Key Takeaways from the Axillary hyperhidrosis Pipeline Report
*
DelveInsight's Axillary Hyperhidrosis pipeline report highlights a dynamic landscape with more than three active companies developing multiple therapeutic candidates for the treatment of axillary hyperhidrosis.
*
In June 2024, the FDA approved Sofdra (sofpironium bromide) topical gel for treating primary axillary hyperhidrosis in patients aged nine and older. Sofdra, an anticholinergic, selectively targets M3 muscarinic receptors in sweat glands to reduce sweat production. This approval represents the first new chemical entity specifically indicated for primary axillary hyperhidrosis, providing a convenient, once-daily, self-administered option for patients who previously had limited treatments.
*
In April 2023, the FDA cleared the Brella SweatControl Patch for adults with primary axillary hyperhidrosis. This single-use, in-office device from Candesant Biomedical employs targeted alkali thermolysis (TAT) technology, generating localized heat through interaction with sweat to decrease gland activity. A single three-minute application can offer sweat reduction for up to four months.
*
Key companies such as GlaxoSmithKline, Medytox, Theravidam, Candesant Biomedical, and others are actively pursuing new therapies to enhance the treatment landscape for axillary hyperhidrosis. Promising pipeline candidates include Sofpironium bromide, ET-01, DMT410, among others.
Axillary hyperhidrosis Overview:
Hyperhidrosis is a disorder characterized by excessive sweating, often starting in childhood or adolescence and sometimes persisting throughout life. Primary hyperhidrosis most commonly affects the palms, soles, underarms (axilla), groin, and area beneath the breasts. The precise cause of axillary hyperhidrosis remains unclear, but it is associated with overactive stimulation of sweat glands by the nervous system. While the sweat glands enlarge and produce excess sweat, this is viewed as a secondary effect. Sweating can be triggered by factors such as anxiety, stress, pain, physical activity, caffeine, or nicotine. In some instances, excessive sweating may affect the entire body or lead to persistent facial redness. The skin in affected regions may appear discolored, soft, cracked, or scaly. Genetics also play a role, with up to 30% of cases reporting a family history. The severity of hyperhidrosis ranges from occasional to continuous daily sweating.
Download the Axillary hyperhidrosis sample report to know in detail about the Axillary hyperhidrosis treatment market [https://www.delveinsight.com/sample-request/axillary-hyperhidrosis-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
Axillary hyperhidrosis Pipeline Analysis
The Axillary hyperhidrosis pipeline insights report 2025, provides insights into:
*
Provides comprehensive insights into key companies developing therapies in the Axillary hyperhidrosis Market.
*
Categorizes Axillary hyperhidrosis therapeutic companies by development stage: early, mid, and late-stage.
*
Highlights major companies involved in targeted therapy development, including both active and inactive (paused/discontinued) projects.
*
Reviews emerging Axillary hyperhidrosis drugs under development based on:
*
Stage of development
*
Axillary hyperhidrosis Route of administration
*
Target receptor
*
Monotherapy vs. combination therapy
*
Axillary hyperhidrosis Mechanism of action
*
Molecular type
*
Offers detailed analysis of:
*
Company-to-company and company-academia collaborations
*
Axillary hyperhidrosis Licensing agreements
*
Funding and investment activities supporting future Axillary hyperhidrosis market advancement.
Unlock key insights into emerging Axillary hyperhidrosis therapies and market strategies here: https://www.delveinsight.com/report-store/axillary-hyperhidrosis-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr
Axillary hyperhidrosis Emerging Drugs
*
Sofpironium bromide: Brickell Biotech Inc.
Sofpironium bromide is an innovative compound under development in the U.S. as a potential once-daily topical therapy for primary axillary hyperhidrosis (excessive underarm sweating). This retro-metabolically engineered anticholinergic works by targeting M3 acetylcholine receptors, aiming to decrease sweat production.
*
ET-01: Eirion Therapeutics
ET-01 is a topical botulinum therapy under development designed to reduce the pain, bruising, and bleeding associated with injectable botulinum treatments. It employs a nanoemulsion-based transdermal delivery system to facilitate absorption of the large botulinum molecule through the skin. ET-01 is currently being evaluated in Phase 2 clinical trials for axillary hyperhidrosis.
*
DMT410: Dermata Therapeutics LLC pipeline
DMT410 represents the second program combining the DMT400 regimen with botulinum toxin. Dermata Therapeutics has conducted a Phase 1 proof-of-concept study evaluating DMT410 alongside BOTOX Registered for treating primary axillary hyperhidrosis. The therapy was generally well tolerated, with most participants showing a reduction in sweating similar to that seen with standard BOTOX Registered injections.
Axillary hyperhidrosis Pipeline Therapeutic Assessment
Axillary hyperhidrosis Assessment by Product Type
- Mono
- Combination
- Mono/Combination
Axillary hyperhidrosis By Stage
- Late-stage products (Phase III)
- Mid-stage products (Phase II)
- Early-stage product (Phase I) along with the details of
- Pre-clinical and Discovery stage candidates
- Discontinued & Inactive candidates
Axillary hyperhidrosis Assessment by Route of Administration
- Oral
- Parenteral
- Intravenous
- Subcutaneous
- Topical
Axillary hyperhidrosis Assessment by Molecule Type
- Recombinant fusion proteins
- Small molecule
- Monoclonal antibody
- Peptide
- Polymer
- Gene therapy
Download sample pages to get an in-depth assessment of the emerging Axillary hyperhidrosis therapies and key Axillary hyperhidrosis companies [https://www.delveinsight.com/sample-request/axillary-hyperhidrosis-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
Table of Contents
1. Report Introduction
2. Executive Summary
3. Axillary hyperhidrosis Current Treatment Patterns
4. Axillary hyperhidrosis - DelveInsight's Analytical Perspective
5. Therapeutic Assessment
6. Axillary hyperhidrosis Late-Stage Products (Phase-III)
7. Axillary hyperhidrosis Mid-Stage Products (Phase-II)
8. Early Stage Products (Phase-I)
9. Pre-clinical Products and Discovery Stage Products
10. Inactive Products
11. Dormant Products
12. Axillary hyperhidrosis Discontinued Products
13. Axillary hyperhidrosis Product Profiles
14. Axillary hyperhidrosis Key Companies
15. Axillary hyperhidrosis Key Products
16. Dormant and Discontinued Products
17. Axillary hyperhidrosis Unmet Needs
18. Axillary hyperhidrosis Future Perspectives
19. Axillary hyperhidrosis Analyst Review
20. Appendix
21. Report Methodology
Request the sample PDF to get detailed insights about the Axillary hyperhidrosis pipeline reports offerings [https://www.delveinsight.com/report-store/axillary-hyperhidrosis-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports Pharma companies by providing comprehensive end-to-end solutions to improve their performance.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Kritika Rehani
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=axillary-hyperhidrosis-pipeline-2025-pioneering-clinical-developments-by-3-global-leaders-delveinsight-highlighting-glaxosmithkline-medytox-theravidam-candesant-biomedical]
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: Nevada
Country: United States
Website: https://www.delveinsight.com/
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Axillary hyperhidrosis Pipeline 2025: Pioneering Clinical Developments by 3+ Global Leaders - DelveInsight | Highlighting GlaxoSmithKline, Medytox, Theravidam, Candesant Biomedical here
News-ID: 4192722 • Views: …
More Releases from ABNewswire
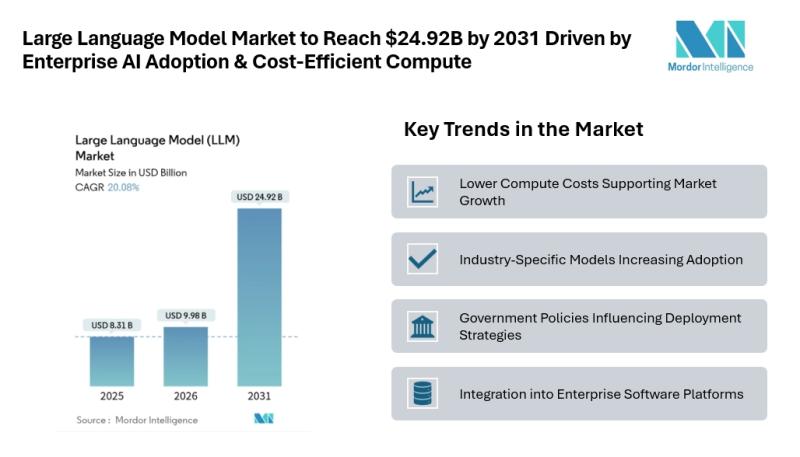
Large Language Model Market to Reach $24.92B by 2031 Driven by Enterprise AI Ado …
Mordor Intelligence has published a new report on the large language model market, offering a comprehensive analysis of trends, growth drivers, and future projections.
Large Language Model Market Outlook
According to Mordor Intelligence, the LLM market size [https://www.mordorintelligence.com/industry-reports/large-language-model-llm-market?utm_source=abnewswire] was valued at USD 8.31 billion in 2025 and is estimated to grow to USD 9.98 billion in 2026, reaching USD 24.92 billion by 2031 at a CAGR of 20.08% during the forecast period.…

Self Employed Tax Software UK: Why Freelancers and Sole Traders Are Switching to …
With Many individuals are seeking software that simplifies tax filing while ensuring full compliance with HMRC requirements. Manual spreadsheets and paper-based calculations are being replaced by real-time, automated systems that give users visibility over their tax position throughout the year. Among the platforms gaining traction is Pie, a UK-based digital tax app built specifically to support self-employed individuals with modern income needs.
LONDON, United Kingdom - February 19, 2026 - Demand…
CivicMail.org Reinvents Postcard Campaigns for Grassroots Advocacy
CivicMail.org aims to bring civic engagement back to basics through the power of pen, paper, and postage.
Image: https://www.abnewswire.com/upload/2026/02/2addd1e9e0381d7e2262e1edbb064123.jpg
CivicMail.org [https://civicmail.org/] has announced its launch to help Americans send real, physical postcards to their elected officials with just a few clicks, delivering personalized messages directly to the desks of decision-makers at the local, state, and federal levels.
Research shows [https://www.concordia.ca/news/stories/2021/09/24/personalized-messages-are-more-likely-to-get-a-response-from-politicians-new-research-finds.html] that physical mail carries more weight with elected officials than petitions, emails, or…

New Children's Story: The Story of Sharin' Bear
A Heartfelt Message Of Courage, Kindness, And The True Meaning Of Giving
A pleasant new story for children, The Story of Sharin' Bear by Sharon Woods , introduces families to a lovable little cub whose journey of bravery and compassion changes him into a representation of sharing for children globally.
Entrenched in adventure, innocence, and emotional growth, this uplifting tale offers an unforgettable reminder that even the smallest acts of kindness can…
More Releases for Axillary
Axillary Crutches Market Size 2024 to 2031.
Market Overview and Report Coverage
Axillary crutches are commonly used mobility aids to assist individuals in walking when they have difficulty bearing weight on their legs or feet. These crutches are designed with padded axillary supports that rest under the arms and provide support while walking.
The Axillary Crutches Market is expected to witness significant growth in the coming years, with a projected CAGR of 12.20% during the forecasted…
Massive Growth in Axillary Crutches Market, 2021-2026
(Portland, United States): Big Market Research newly added a research report on the Axillary Crutches Market which represents a study for the period from 2021 to 2026. The research study provides a near look at the market scenario and dynamics impacting its growth. This report highlights the crucial developments along with other events happening in the market which are marking on the growth and opening doors for future growth in…
Axillary Crutches Market - Size, Share, Outlook, and Opportunity
Axillary crutches are the mobility aids used by individuals suffering from walking disability, factures, paralysis, and leg injuries. Axillary crutches transfer the weight from legs to the arms/shoulders and support the movement of individuals who are unable to walk due to some disability. Axillary crutches are lightweight and they are available in various materials, which includes aluminum, stainless steel, and wooden. Axillary crutches are adjustable in size and can be…
Axillary Crutches Market - Size, Share, Outlook, and Opportunity Analysis, 2018 …
Axillary crutches are the mobility aids used by individuals suffering from walking disability, factures, paralysis, and leg injuries. Axillary crutches transfer the weight from legs to the arms/shoulders and support the movement of individuals who are unable to walk due to some disability. Axillary crutches are lightweight and they are available in various materials, which includes aluminum, stainless steel, and wooden. Axillary crutches are adjustable in size and can be…
Global Axillary Crutches Market: Top Players - Invacare, Stander, Sizewise
Latest industry research report on: Global Axillary Crutches Market : Industry Size, Share, Research, Reviews, Analysis, Strategies, Demand, Growth, Segmentation, Parameters, Forecasts
Request For Sample Report @https://www.marketresearchreports.biz/sample/sample/1001065
This report studies sales (consumption) of Axillary Crutches in Global market, especially in United States, China, Europe and Japan, focuses on top players in these regions/countries, with sales, price, revenue and market share for each player in these regions, covering
Millennial Medical
Ergoactives
Cardinal Health
AMG Medical Inc/Airgo
Carex
Graham Field
Nova…
Global Axillary Crutches Market Research Report 2017
Report Hive Market Research Released a New Research Report of 102 pages on Title " Global Axillary Crutches Market Research Report 2017 "with detailed Analysis, Forecast and Strategies.
In this report, the global Axillary Crutches market is valued at USD XX million in 2016 and is expected to reach USD XX million by the end of 2022, growing at a CAGR of XX% between 2016 and 2022.
Geographically, this report is segmented…
