Press release
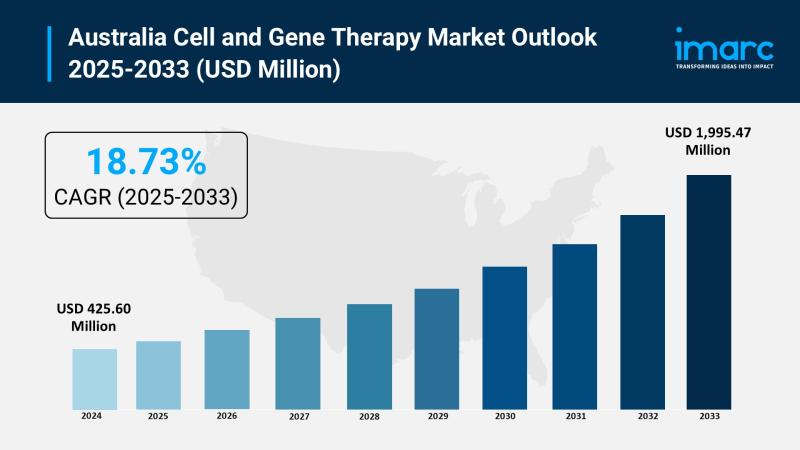
Australia Cell and Gene Therapy Market to Reach USD 1,995.47 Million by 2033
The latest report by IMARC Group, "Australia Cell and Gene Therapy Market Size, Share, Trends and Forecast by Therapy Type, Indication, Delivery Mode, End User, and Region, 2025-2033," provides an in-depth analysis of the Australia cell and gene therapy market. The report also includes competitor and regional analysis, along with a breakdown of segments within the industry. The Australia cell and gene therapy market size reached USD 425.60 million in 2024 and is projected to grow to USD 1,995.47 million by 2033, exhibiting an exceptional growth rate of 18.73% during the forecast period.Report Attributes and Key Statistics:
Base Year: 2024
Forecast Years: 2025-2033
Historical Years: 2019-2024
Market Size in 2024: USD 425.60 Million
Market Forecast in 2033: USD 1,995.47 Million
Growth Rate (2025-2033): 18.73%
Australia Cell and Gene Therapy Market Overview:
The Australia cell and gene therapy market is experiencing exceptional growth driven by increased government funding, cross-border collaborations, and rising clinical trial activity supporting early-stage innovation and advanced manufacturing for treatments targeting rare diseases and regenerative health conditions. The market demonstrates remarkable momentum with strategic developments including the Australian Government's USD 2 million allocation to Mirugen Pty Ltd under the CURéator+ program in December 2024 for vision restoration gene therapy. Strategic international partnerships are transforming the sector, exemplified by INmune Bio's collaboration with UK-based Cell and Gene Therapy Catapult in April 2025 to scale CORDStrom therapy production, while Cell Therapies Pty Ltd and Xellera Therapeutics formed partnerships expanding access across Asia-Pacific region. The sector benefits from focus on regenerative treatment innovation, expansion of clinical manufacturing capacity, and advances in personalized medicine addressing unique genetic patterns.
Request For Sample Report: https://www.imarcgroup.com/australia-cell-gene-therapy-market/requestsample
Australia Cell and Gene Therapy Market Trends:
• Regenerative Treatment Innovation advancing through USD 2 million government funding to Mirugen Pty Ltd for vision restoration gene therapy under CURéator+ program demonstrating commitment to breakthrough applications
• International Manufacturing Partnerships expanding through INmune Bio's collaboration with UK Cell and Gene Therapy Catapult scaling CORDStrom production for RDEB rare genetic skin condition
• Asia-Pacific Expansion accelerating through Cell Therapies Pty Ltd and Xellera Therapeutics strategic partnership enhancing cell and gene therapy access across Australia and Hong Kong
• Personalized Medicine Advancement targeting unique genetic patterns through improved sequencing, biomarker identification, and diagnostic technologies enabling patient-specific therapy matching
• Clinical Trial Activity Growth demonstrating 74% non-oncology indication trials and 57% oncology gene therapy trials reflecting diversified therapeutic development pipeline
• Digital Integration implementing digital tools for treatment monitoring and expanding collaborations between biotech firms and academic institutions driving innovation
• Manufacturing Capacity Expansion establishing scalable production systems supporting multinational therapeutic development programs and commercial supply preparation
Australia Cell and Gene Therapy Market Drivers:
• Government Funding Support providing substantial investment through programs like CURéator+ with USD 2 million allocation to Mirugen Pty Ltd advancing regenerative medicine capabilities
• Rising Chronic Disease Prevalence creating substantial demand for curative solutions targeting cancer, rare genetic conditions, and diseases with limited traditional treatment options
• Cross-Border Collaboration Growth enhancing development capacity through international partnerships enabling technology transfer, expertise sharing, and expanded clinical trial access
• Regulatory Framework Enhancement streamlining approval processes for breakthrough treatments ensuring faster patient access while maintaining safety and quality standards
• Technological Innovation advancing genetic sequencing, molecular diagnostics, stem cell research, and manufacturing processes making therapies more targeted and commercially viable
• Patient Demand Increase driving market expansion as awareness grows and treatment accessibility improves for long-term curative outcomes over traditional therapies
• Research Infrastructure Investment supporting biotech companies, academic institutions, and healthcare providers through enhanced facilities, equipment, and collaborative programs
Market Challenges:
• High Development Costs requiring substantial capital investment for research, clinical trials, manufacturing infrastructure, and regulatory compliance affecting market entry barriers
• Complex Manufacturing Requirements demanding sophisticated production capabilities, quality control systems, and specialized facilities for cell and gene therapy products
• Regulatory Complexity navigating evolving approval pathways, safety requirements, and quality standards specific to advanced biological therapies requiring specialized expertise
• Limited Clinical Evidence requiring extensive long-term studies demonstrating safety and efficacy for novel therapies addressing previously untreatable conditions
• Supply Chain Dependencies managing complex logistics for temperature-sensitive products, specialized equipment, and raw materials affecting production reliability
• Skilled Workforce Shortage addressing limited availability of professionals experienced in cell and gene therapy development, manufacturing, and clinical application
• Market Access Challenges overcoming high treatment costs, reimbursement limitations, and healthcare system integration barriers affecting patient accessibility
Market Opportunities:
• Rare Disease Focus developing targeted therapies for orphan diseases with unmet medical needs offering significant market potential and regulatory incentives
• Regional Hub Development establishing Australia as Asia-Pacific center for cell and gene therapy through manufacturing partnerships and technology transfer initiatives
• Commercialization Support leveraging government programs transitioning scientific research into market-ready products with curative potential for irreversible conditions
• Digital Health Integration implementing AI, machine learning, and digital monitoring tools optimizing treatment outcomes and patient management systems
• Academic-Industry Partnerships expanding collaboration between research institutions and biotech companies accelerating innovation and clinical translation
• Export Market Potential serving regional markets through advanced manufacturing capabilities, quality standards, and strategic geographic positioning
• Precision Medicine Expansion developing customized therapies based on individual genetic profiles, biomarkers, and disease characteristics improving treatment effectiveness
Browse The Full Report with TOC & List of Figures: https://www.imarcgroup.com/australia-cell-gene-therapy-market
Australia Cell and Gene Therapy Market Segmentation:
By Therapy Type:
• Cell Therapy (Stem Cell: Pluripotent Stem Cell, Cancer Stem Cell, Adult Stem Cell; Non-Stem Cell: T Cells, Natural Killer, Others)
• Gene Therapy
By Indication:
• Cardiovascular Disease
• Oncology Disorder
• Genetic Disorder
• Infectious Disease
• Neurological Disorder
• Others
By Delivery Mode:
• In-Vivo
• Ex-Vivo
By End User:
• Hospitals
• Cancer Care Centers
• Pharmaceutical & Biotechnology Companies
• Others
By Regional Distribution:
• Australia Capital Territory & New South Wales
• Victoria & Tasmania
• Queensland
• Northern Territory & Southern Australia
• Western Australia
Australia Cell and Gene Therapy Market News:
August 2025: Cell and Gene Therapy CDMO market projected to grow at 27% CAGR with Cell Therapies Pty Ltd and Xellera Therapeutics partnership accelerating development across Asia-Pacific region including Australia and Hong Kong through enhanced GMP capabilities.
April 2025: INmune Bio partnered with UK-based Cell and Gene Therapy Catapult to scale CORDStrom production for rare genetic skin condition RDEB, positioning Australia as key node in global therapeutic development with expanded manufacturing support.
December 2024: Australian Government allocated USD 2 million to Mirugen Pty Ltd under CURéator+ program for vision restoration gene therapy, reflecting commitment to regenerative medicine capabilities and breakthrough treatment development.
July 2025: Global genome editing market expected to grow from $10.8 billion in 2025 to $23.7 billion by 2030 at 16.9% CAGR, benefiting Australia's position in advanced biological therapy development and innovation.
Key Highlights of the Report:
• Comprehensive market analysis projecting exceptional growth from $425.60 million in 2024 to $1,995.47 million by 2033
• Detailed examination of government support through USD 2 million CURéator+ program funding to Mirugen Pty Ltd for vision restoration therapy
• Strategic assessment of international partnerships including INmune Bio-Cell and Gene Therapy Catapult collaboration scaling rare disease treatments
• In-depth analysis of Asia-Pacific expansion through Cell Therapies Pty Ltd and Xellera Therapeutics partnership enhancing regional access
• Regional market evaluation covering five major zones with diverse healthcare infrastructure and research capabilities
• Technology advancement insights highlighting personalized medicine, digital integration, and manufacturing capacity expansion
• Clinical trial activity assessment revealing 74% non-oncology and 57% oncology gene therapy trials demonstrating pipeline diversity
Frequently Asked Questions (FAQs):
Q1: What are the primary factors driving Australia's cell and gene therapy market growth to $1,995.47 million by 2033?
A1: The market is driven by increased government funding including USD 2 million CURéator+ program allocation, cross-border collaborations with UK Cell and Gene Therapy Catapult and rising clinical trial activity. Focus on regenerative treatment innovation, personalized medicine advances, and expanding manufacturing capacity contribute to the exceptional 18.73% growth rate during the forecast period.
Q2: How are international partnerships influencing market development?
A2: International partnerships are transforming the sector through collaborations like INmune Bio's partnership with UK-based Cell and Gene Therapy Catapult scaling CORDStrom production, and Cell Therapies Pty Ltd-Xellera Therapeutics alliance expanding access across Asia-Pacific. These partnerships enable technology transfer, expertise sharing, and position Australia as a key node in global therapeutic development networks.
Q3: What role does government support play in market expansion?
A3: Government support is fundamental through initiatives like the CURéator+ program providing USD 2 million funding to Mirugen Pty Ltd for vision restoration gene therapy. This support reflects commitment to regenerative medicine capabilities, commercialization pathways, and building therapeutic pipelines with long-term curative potential while encouraging private-sector participation in early-stage development.
Note: If you require specific information not currently within the scope of the report, we can provide it as part of the customization.
Ask an analyst for your customized sample: https://www.imarcgroup.com/request?type=report&id=32737&flag=C
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel. No.: (D) +91 120 433 0800
Americas: +1 201-971-6302
About Us:
IMARC Group is a global management consulting firm that helps the world's most ambitious changemakers to create a lasting impact. The company provides a comprehensive suite of market entry and expansion services. IMARC offerings include market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Australia Cell and Gene Therapy Market to Reach USD 1,995.47 Million by 2033 here
News-ID: 4191485 • Views: …
More Releases from IMARC Group

Base Oil Price Trend Analysis: Current Prices, Index & Forecast 2026
The Base Oil Price Index has shown notable fluctuations in late 2025 and early 2026 due to shifts in crude oil markets, refinery output, and global demand. Tracking the price of Base Oil is essential for lubricants manufacturers, industrial users, and traders seeking insights into market dynamics. This report provides a comprehensive overview of Base Oil Prices, including historical data, price trends, forecasts for 2026, and regional variations. By analyzing…
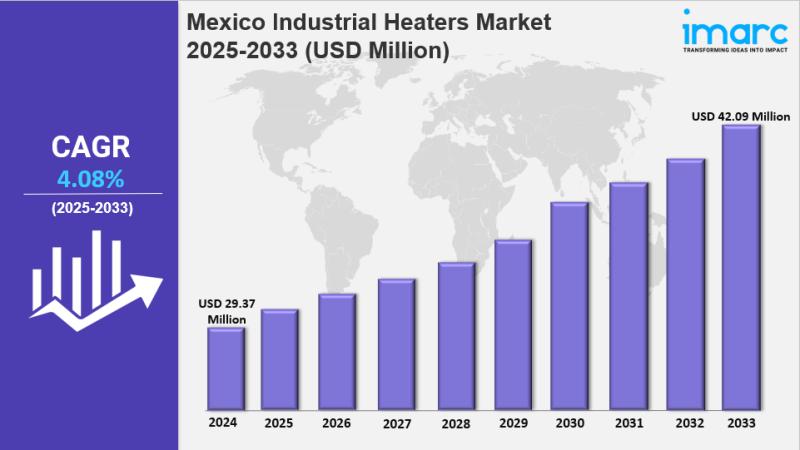
Mexico Industrial Heaters Market Share, Size, In-Depth Insights, Trends and Fore …
IMARC Group has recently released a new research study titled "Mexico Industrial Heaters Market Size, Share, Trends and Forecast by Product, Technology, End User, and Region, 2025-2033", offers a detailed analysis of the market drivers, segmentation, growth opportunities, trends and competitive landscape to understand the current and future market scenarios.
Market Overview
The Mexico industrial heaters market was valued at USD 29.37 Million in 2024 and is expected to reach USD 42.09…
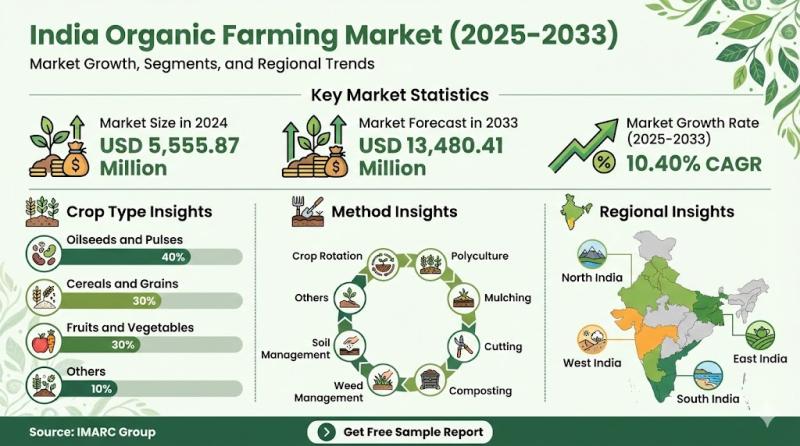
India Organic Farming Market 2025-2033: $13,480.41Mn Industry Growth, Trends & S …
Source: IMARC Group | Category: Agriculture | Author: Tarang
Report Introduction
According to IMARC Group's latest report titled "India Organic Farming Market Size, Share, Trends and Forecast by Crop Type, Method, and Region, 2025-2033", this study offers a granular analysis of the industry's rapid transition towards sustainable agricultural practices and chemical-free food production. The study offers a profound analysis of the industry, encompassing market share, size, India organic farming market growth factors,…

Electric Vehicle Manufacturing Plant DPR - 2026, Machinery Cost, ROI, and Market …
The global automotive industry stands at a historic inflection point as the world accelerates its transition from conventional internal combustion engines to electric mobility. Electric vehicles represent a transformative shift in personal and commercial transportation, offering zero tailpipe emissions, significantly lower operating costs, and quieter operation compared to traditional gasoline and diesel vehicles. As tightening emission regulations, rising fuel prices, government incentives for clean mobility, expanding charging infrastructure, and increasing…
More Releases for Cell
Cell Sorting Market Accelerates as Cell Therapy, Immuno-Oncology & Single-Cell R …
The rising focus on precision medicine, immunotherapy, and advanced cell-based research is driving the global cell sorting market into a high-growth phase. With expanding applications in stem cell therapy, CAR-T manufacturing, cancer immunology, and single-cell genomics, demand for accurate, high-purity cell isolation systems is stronger than ever. This release highlights key market trends, segmentation insights, technological innovations, and the factors shaping the future of cell sorting.
Download Full PDF Sample Copy…
Cell Isolation Cell Separation Market Size Analysis by Application, Type, and Re …
According to Market Research Intellect, the global Cell Isolation Cell Separation market under the Internet, Communication and Technology category is expected to register notable growth from 2025 to 2032. Key drivers such as advancing technologies, changing consumer behavior, and evolving market dynamics are poised to shape the trajectory of this market throughout the forecast period.
The market for cell isolation and separation is expanding rapidly as a result of sophisticated biotechnological…
Cell Free Protein Synthesis Market Beyond the Cell: Revolutionizing Protein Prod …
Cell-Free Protein Synthesis Market to reach over USD 457.13 Mn by the year 2031 - Exclusive Report by InsightAce Analytic
"Cell-Free Protein Synthesis Market" in terms of revenue was estimated to be worth $265.94 Mn in 2023 and is poised to reach $457.13 Mn by 2031, growing at a CAGR of 7.20% from 2024 to 2031 according to a new report by InsightAce Analytic.
Request for free Sample Pages: https://www.insightaceanalytic.com/request-sample/1445
Current…
Cell Expansion Market - Expand the Boundaries of Cell Therapy: Redefine Cell Exp …
Newark, New Castle, USA: The "Cell Expansion Market" provides a value chain analysis of revenue for the anticipated period from 2022 to 2030. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors
Cell Expansion Market: https://www.growthplusreports.com/report/cell-expansion-market/7939
This latest report researches the industry structure, sales, revenue,…
Global GMP Cell Banking Market By Type - Mammalian Cell, Microbial Cell, Insect …
Researchmoz added Most up-to-date research on "Global GMP Cell Banking Market By Type - Mammalian Cell, Microbial Cell, Insect Cell and Others" to its huge collection of research reports.
This report researches the worldwide GMP Cell Banking market size (value, capacity, production and consumption) in key regions like North America, Europe, Asia Pacific (China, Japan) and other regions.
This study categorizes the global GMP Cell Banking breakdown data by manufacturers, region, type…
Cell Culture Market Size, Cell Culture Market Share, Cell Culture Market Trends …
According to a new research published by Polaris Market Research the global cell culture market is anticipated to reach more than USD 49 billion by 2026. Cell culture is a rapidly emerging as an implement for analyzing and treating various disease such as Alzheimer’s and cancer.
Request for Sample of This Research Report @ https://bit.ly/2D7pZ5u
Top Key Players: -
Becton,
Dickinson and Company
Biospherix
EMD Millipore
Eppendorf AG
Merck KGaA
Sartorius AG
VWR International
Cell culture is a rapidly emerging…