Press release
Nephrotic Syndrome Market Poised for Significant Growth as Novel Therapies Drive Market Forward | DelveInsight
DelveInsight's comprehensive analysis reveals the Nephrotic Syndrome therapeutics market reached USD 401 million in 2022 across the 7MM, with emerging therapies including FDA-approved Furoscix and promising pipeline drugs Atacicept and Atrasentan driving substantial growth during the 2023-2034 forecast period.Key Findings
*
Market size projection: As per DelveInsight's analysis, the total market size of Nephrotic Syndrome in the 7MM is expected to witness consistent growth throughout the forecast period (2023-2034), driven by the launch of emerging therapies and expanded treatment indications.
*
Market Drivers: Key market drivers of Nephrotic Syndrome include the unmet medical needs in current treatment approaches, increasing prevalence of chronic kidney disease and related disorders, advancement in targeted biologics and monoclonal antibodies, expanding pipeline of novel therapeutic approaches, and regulatory approvals for innovative treatment options.
*
Epidemiology: The report provides the total Nephrotic Syndrome potential pool with DelveInsight's analyst projecting that among the total diagnosed prevalent cases in 7MM, approximately 34% of cases were from the US. In 2022, the EU4 and the UK accounted for nearly 367 thousand diagnosed prevalent cases, while the US had approximately 268k diagnosed cases expected to increase at an estimated CAGR throughout the study period (2020-2034).
*
Key companies: Leading Nephrotic Syndrome companies include GlaxoSmithKline, Goldfinch Bio Inc., Travere Therapeutics Inc., Aurinia Pharmaceuticals, Bristol-Myers Squibb, ChemoCentryx, SynAct Pharma AB, Vera Therapeutics Inc., Vertex Pharmaceuticals, Chinook Therapeutics (Novartis), Boehringer Ingelheim, Hoffmann-La Roche, Biogen, and others.
*
Pipeline assets: Some of the key Nephrotic Syndrome drugs in the pipeline include Atacicept (Vera Therapeutics), Atrasentan (Chinook Therapeutics/Novartis), Gazyva/Gazyvaro (obinutuzumab) (Hoffmann-La Roche), Zigakibart (BION-1301), and others.
*
Recent developments:
*
In March 2025, the FDA expanded the approval of Furoscix (furosemide injection) to include the treatment of edema in patients with chronic kidney disease, including nephrotic syndrome.
*
The FDA also accepted Hoffmann-La Roche's supplemental Biologics License Application for Gazyva/Gazyvaro (obinutuzumab) for lupus nephritis treatment, with a decision expected by October 2025.
*
Additionally, Travere Therapeutics announced FDA acceptance of its sNDA for FILSPARI (sparsentan) in FSGS, with a PDUFA target action date of January 13, 2026.
Discover more recent advancements in the Nephrotic Syndrome landscape @ Nephrotic Syndrome Recent Developments [https://www.delveinsight.com/sample-request/nephrotic-syndrome-market?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=xpr].
Nephrotic Syndrome Market Intelligence
The Nephrotic Syndrome treatment market represents a significant therapeutic area addressing a complex kidney disorder characterized by massive proteinuria, hypoalbuminemia, hyperlipidemia, and edema. The market reached approximately USD 401 million in 2022 across the 7MM, consisting primarily of corticosteroids, calcineurin inhibitors, ACE inhibitors/ARBs, and immunosuppressants. The United States dominated the market with approximately 34% of total diagnosed prevalent cases, while Japan represented approximately 16% of the total market in 2022, projected to increase at a substantial CAGR during the study period.
According to DelveInsight's Nephrotic Syndrome Market Insights, Epidemiology, and Market Forecast report [https://www.delveinsight.com/report-store/nephrotic-syndrome-market?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=xpr], the market growth projections during the forecast period is driven by the emergence of new and effective treatments targeting specific molecular pathways. Currently, there are no authorized therapies specifically designed for treating nephrotic syndrome, with predominant treatment strategies often involving off-label approaches. This represents a significant unmet medical need that emerging therapies are positioned to address.
Key market drivers include the increasing recognition of nephrotic syndrome's impact on patient quality of life, advances in understanding disease pathophysiology, development of targeted biologics and monoclonal antibodies, and regulatory support for orphan drug development. The geographical analysis reveals varying prevalence patterns, with Germany having the highest diagnosed prevalent population in EU4 and the UK at about 98k cases, followed by the UK and France in 2022, while Spain had the lowest diagnosed prevalent population.
Epidemiologically, nephrotic syndrome affects diverse patient populations across primary and secondary glomerulonephropathies. In the US, approximately 214k cases were attributed to primary glomerulonephropathies and 54k cases to secondary glomerulonephropathies in 2022, with cases expected to increase during the forecast period (2023-2034). The 7MM breakdown shows significant regional variations in disease burden and treatment approaches, influencing market dynamics and therapeutic access patterns.
For a deeper analysis of the evolving Nephrotic Syndrome market, download our free sample report [https://www.delveinsight.com/sample-request/nephrotic-syndrome-market?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=xpr].
Nephrotic Syndrome Competitive Landscape
The competitive landscape encompasses established pharmaceutical companies and emerging biotechnology firms developing innovative therapeutic approaches. Key players include GlaxoSmithKline with their anti-CD20 monoclonal antibody programs, Vera Therapeutics developing Atacicept for B-cell targeted therapy, and Chinook Therapeutics (acquired by Novartis) advancing Atrasentan as an endothelin A receptor antagonist. Hoffmann-La Roche leads with Gazyva/Gazyvaro (obinutuzumab) showing promising results in lupus nephritis clinical trials.
The Nephrotic Syndrome clinical pipeline activity demonstrates robust development across multiple therapeutic approaches, with phase II and III studies evaluating novel mechanisms of action. The pipeline includes monoclonal antibodies targeting APRIL and BAFF pathways, endothelin receptor antagonists, and dual endothelin-angiotensin receptor antagonists. Recent clinical developments include the successful phase III REGENCY trial for obinutuzumab in lupus nephritis, demonstrating superior renal response rates compared to standard therapy alone.
Clinical landscape developments further include include significant regulatory milestones, such as the March 2025 FDA approval expanding Furoscix (furosemide injection) indications to include edema treatment in chronic kidney disease patients, including those with nephrotic syndrome. This subcutaneous loop diuretic provides IV-equivalent diuresis through a wearable infusor system, representing an important advancement in patient care delivery.
Development milestones include Hoffmann-La Roche's obinutuzumab achieving positive phase III REGENCY trial results in lupus nephritis, with the FDA accepting their supplemental BLA submission and assigning an October 2025 decision timeline. The trial demonstrated that 46.4% of patients receiving obinutuzumab plus standard therapy achieved complete renal response compared to 33.1% receiving placebo plus standard therapy.
Commercial arrangements reflect strategic partnerships and acquisitions shaping the competitive landscape. The Novartis acquisition of Chinook Therapeutics strengthened their nephrology portfolio, while companies like Travere Therapeutics continue advancing sparsentan through regulatory processes, with FDA acceptance of their FSGS sNDA and a January 2026 PDUFA date.
Pipeline diversity encompasses various therapeutic modalities, including Zigakibart (BION-1301), along with established therapies being repositioned for nephrotic syndrome applications. The development focus on targeted biologics, particularly monoclonal antibodies and receptor antagonists, represents a shift toward precision medicine approaches in nephrology.
Delve deeper into the major and specialised companies in the Nephrotic Syndrome market @ Nephrotic Syndrome Competitive Landscape [https://www.delveinsight.com/sample-request/nephrotic-syndrome-market?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=xpr].
Nephrotic Syndrome Market Drivers and Barriers
The unmet medical needs represent the primary market driver, as currently no authorized therapies are specifically designed for treating nephrotic syndrome. Predominant treatment strategies involve off-label approaches using corticosteroids, cyclophosphamide, cyclosporine, diuretics, and ACE inhibitors or ARBs, highlighting the urgent need for targeted therapeutic options.
Other market drivers include advancing understanding of nephrotic syndrome pathophysiology, leading to targeted therapeutic development. The emergence of novel biologics and receptor antagonists targeting specific molecular pathways offers potential for improved patient outcomes. Regulatory support through breakthrough therapy designations and orphan drug programs facilitates accelerated development timelines. Additionally, increasing awareness of chronic kidney disease burden and the growing aging population contribute to market expansion potential.
Market barriers include the complex and heterogeneous nature of nephrotic syndrome, making clinical trial design challenging. High development costs associated with rare disease therapeutics, regulatory uncertainties, and the need for long-term safety data present significant challenges. Limited patient populations for clinical trials and varying disease presentation across different underlying etiologies complicate drug development efforts.
Download the Nephrotic Syndrome Market report to understand which other factors are driving the therapeutic market @ Nephrotic Syndrome Market Trends [https://www.delveinsight.com/sample-request/nephrotic-syndrome-market?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=xpr].
Conclusion
The Nephrotic Syndrome therapeutics market stands at a pivotal juncture, with substantial growth potential driven by emerging therapies addressing significant unmet medical needs. The recent FDA approvals and regulatory progress for drugs like Furoscix, obinutuzumab, and sparsentan demonstrate advancing therapeutic options for patients facing this challenging kidney disorder. As the market transitions from primarily off-label treatments to targeted, disease-specific therapies, stakeholders can anticipate continued expansion throughout the forecast period. The robust pipeline of novel biologics and targeted therapies, combined with increasing understanding of disease mechanisms, positions the market for transformative growth while addressing the critical healthcare needs of nephrotic syndrome patients across the 7MM.
Scope of the Nephrotic Syndrome Market Report
*
Comprehensive insight into Nephrotic Syndrome epidemiology segments and forecasts, future growth potential of diagnosis rate, disease progression, and treatment guidelines
*
All-inclusive account of current and emerging Nephrotic Syndrome therapies and elaborative profiles of late-stage and prominent therapies impacting the current treatment market landscape
*
Detailed review of historical and forecasted Nephrotic Syndrome drugs market size, market share by therapies, detailed assumptions, and rationale behind the approach covering 7MM drug outreach
*
Patient-based Nephrotic Syndrome Market Forecasting providing edge while understanding treatment algorithms, drug uptake, and market forecast assumptions
*
Coverage of Therapeutic Approaches, Pipeline Analysis, Drugs Market Size, Market Trends, and existing and future market opportunities
*
11 years Market Forecast across 7MM Coverage with Epidemiology Segmentation, Key Cross Competition, and Conjoint Analysis
*
Current Treatment Market Practices, Unmet Needs, Pipeline Product Profiles, and Market Attractiveness through Qualitative Analysis including SWOT and Conjoint Analysis
Table of Contents
1. Key Insights
2. Executive Summary of Nephrotic Syndrome
3. Competitive Intelligence Analysis for Nephrotic Syndrome
4. Nephrotic Syndrome Market Overview at a Glance
5. Nephrotic Syndrome: Disease Background and Overview
6. Nephrotic Syndrome Patient Journey
7. Nephrotic Syndrome Epidemiology and Patient Population
8. Treatment Algorithm, Current Treatment, and Medical Practices
9. Nephrotic Syndrome Unmet Needs
10. Key Endpoints of Nephrotic Syndrome Treatment
11. Nephrotic Syndrome Marketed Products
12. Nephrotic Syndrome Emerging Therapies
13. Nephrotic Syndrome: Seven Major Market Analysis
14. Attribute analysis
15. 7MM: Market Outlook
16. Access and Reimbursement Overview of Nephrotic Syndrome
17. KOL Views
18. Nephrotic Syndrome Market Drivers
19. Nephrotic Syndrome Market Barriers
20. Appendix
21. DelveInsight Capabilities
22. Disclaimer
23. About DelveInsight
About DelveInsight
DelveInsight is a leading market research and consulting firm specializing in disease-specific insights and therapeutic market analysis. Their reports integrate real-world data, clinical trial findings, and expert interviews to deliver comprehensive industry intelligence.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Shruti Thakur
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=nephrotic-syndrome-market-poised-for-significant-growth-as-novel-therapies-drive-market-forward-delveinsight]
Phone: 09650213330
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: NV
Country: United States
Website: https://www.delveinsight.com/
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Nephrotic Syndrome Market Poised for Significant Growth as Novel Therapies Drive Market Forward | DelveInsight here
News-ID: 4190177 • Views: …
More Releases from ABNewswire
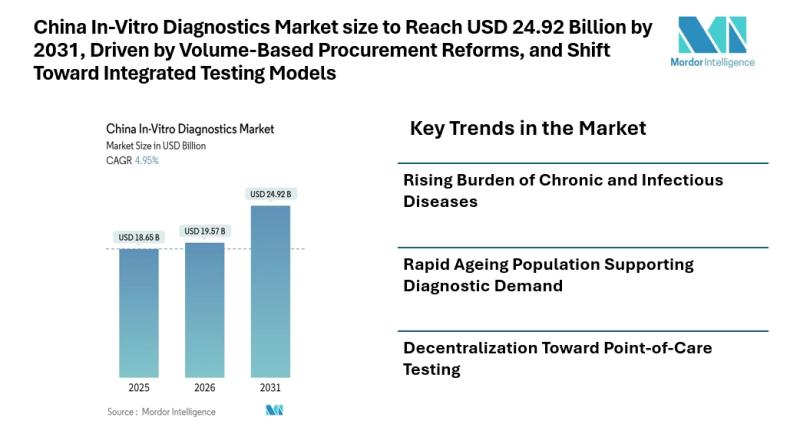
China In-Vitro Diagnostics Market size to Reach USD 24.92 Billion by 2031, Drive …
Mordor Intelligence has published a new report on the china in-vitro diagnostics market, offering a comprehensive analysis of trends, growth drivers, and future projections.
Introduction
According to Mordor Intelligence, the china in-vitro diagnostics market size [https://www.mordorintelligence.com/industry-reports/china-in-vitro-diagnostics-market?utm_source=abnewswire] is projected to reach USD 24.92 billion by 2031, growing from USD 19.57 billion in 2026 at a CAGR of 4.95% during the forecast period. The china in-vitro diagnostics market size reflects steady expansion supported by…

Hyaluronic Acid Market Size to Reach USD 4.07 Billion by 2030 - Mordor Intellige …
Mordor Intelligence has released an in-depth analysis of the hyaluronic acid market, outlining expanding cosmetic, orthopedic, and pharmaceutical applications driving global demand.
Hyaluronic Acid Market Overview
According to Mordor Intelligence, the global hyaluronic acid market size [https://www.mordorintelligence.com/industry-reports/hyaluronic-acid-market?utm_source=abnewswire] reached USD 2.84 billion in 2025 and is projected to grow to USD 4.07 billion by 2030, registering a CAGR of 7.46% during the forecast period.
The strong hyaluronic acid market growth is supported by:
* Increasing…

Scott Bryant Unveils Moon Valley's "Best Value" Listing in Hillcrest East; Signa …
Bryant Real Estate Leverages Data-Driven Performance Metrics to Position New Hillcrest East Property as the Region's Premier Investment Opportunity
PHOENIX, AZ - Scott Bryant, Founder and Team Leader of Bryant Real Estate and a top-performing agent with Keller Williams, has announced the debut of a landmark listing in the Hillcrest East subdivision of Moon Valley. Positioned as "Moon Valley's Best Deal," the property is being introduced at a strategic price point…

Jennifer Rollin Named Best Individual Therapist in Best of Bethesda Awards
Bethesda, MD, USA - Jennifer Rollin, LCSW-C, eating disorder therapist and founder of The Eating Disorder Center, has been named Best Individual Therapist in the 2025 Best of Bethesda Awards. She was selected from among therapists across Montgomery County, Maryland and Upper Northwest Washington, D.C., an honor that reflects both community support and her longstanding commitment to helping individuals recover from eating disorders.
Jennifer Rollin provides eating disorder therapy [https://www.theeatingdisordercenter.com/eatingdisordertherapyrockvilleservices.html] in…
More Releases for Nephrotic
Nephrotic Syndrome Market Detailed Industry Report Analysis 2025-2034
Introduction
Nephrotic syndrome is a clinical condition characterized by excessive protein loss in urine, low blood protein levels, high cholesterol, and swelling, often caused by kidney diseases such as minimal change disease, focal segmental glomerulosclerosis (FSGS), and membranous nephropathy. Affecting both children and adults, it is a major contributor to global kidney disease burden and often progresses to chronic kidney disease (CKD) or end-stage renal disease (ESRD) if not managed effectively.
With…
Nephrotic Syndrome Market Positioned for Accelerated Development Through 2034, D …
DelveInsight's "Nephrotic Syndrome Market Insights, Epidemiology, and Market Forecast-2034′′ report offers an in-depth understanding of the Nephrotic Syndrome, historical and forecasted epidemiology as well as the Nephrotic Syndrome market trends in the United States, EU4 (Germany, Spain, Italy, France) the United Kingdom and Japan.
The latest healthcare forecast report provides an in-depth analysis of Nephrotic Syndrome, offering comprehensive insights into the Nephrotic Syndrome revenue trends, prevalence, and treatment landscape. The…
Nephrotic Syndrome Treatment Market Size, Share, Growth, Trends, and Forecast 20 …
July 2025 | By Shweta Raskar, Business Development Specialist at Prophecy Market Insights
Prophecy Market Insights has recently published a comprehensive research report on the Nephrotic Syndrome Treatment Market, offering in-depth insights into key growth dynamics, future opportunities, and competitive landscape shaping the market from 2025 to 2035. The report spans 145+ pages, delivering valuable intelligence for pharmaceutical companies, healthcare providers, investors, and policymakers.
📎 Get a Free Sample Report Here:
https://www.prophecymarketinsights.com/market_insight/Insight/request-sample/5788
🔑 Key…
Nephrotic Syndrome Market Outlook 2025-2034: Key Trends, Growth Drivers, and Mar …
How Are the key drivers contributing to the expansion of the nephrotic syndrome market?
The increasing incidence of kidney diseases is anticipated to drive the growth of the nephrotic syndrome market. Kidney diseases, which impair the kidneys' ability to filter waste from the blood, are rising due to factors like diabetes, hypertension, obesity, and aging populations. Nephrotic syndrome, which causes damage to the kidneys' glomeruli and impairs filtration, contributes to the…
Nephrotic Syndrome Diagnostics & Therapeutics Market Demand Analysis, Growth, Tr …
The global nephrotic syndrome diagnostics & therapeutics market is anticipated to grow at a CAGR of around 5.6% during the forecast period (2024-2031). The key factor to drive the market growth includesthe increasing prevalence of diabetes that results in increased chances of nephrotic syndrome. For instance, In the European Union, approximately 32.3 million adults were diagnosed with diabetes in 2019, up from an estimated 16.8 million adults in 2000. In…
Focal Segmental Glomerulosclerosis (FSGS) Therapeutics Market - Transforming Kid …
Newark, New Castle, USA: The "Focal Segmental Glomerulosclerosis (FSGS) Therapeutics Market" provides a value chain analysis of revenue for the anticipated period from 2023 to 2031. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors.
Focal Segmental Glomerulosclerosis (FSGS) Therapeutics Market: https://www.growthplusreports.com/report/focal-segmental-glomerulosclerosis-fsgs-therapeutics-market/8837
This latest report…
