Press release
GMP Storage Market to See Steady Gains with Rising Drug Manufacturing Activities - Persistence Market Research
The Good Manufacturing Practice (GMP) storage market plays a pivotal role in the pharmaceutical, biotechnology, and healthcare industries. With a compound annual growth rate (CAGR) of 5.6% from 2022 to 2032, the market is set to reach a value of US$ 10.2 billion by 2032, up from approximately US$ 5.7 billion at the end of 2021. The increasing demand for temperature-sensitive biologics and advancements in cold chain technologies are key contributors to this impressive market growth. This article provides a detailed overview of the GMP storage market, including market statistics, growth drivers, key market segments, and regional trends, as well as insights into competitive strategies.Get a Sample Copy of Research Report (Use Corporate Mail id for Quick Response): https://www.persistencemarketresearch.com/samples/33113
Overview of the Market
The GMP storage market is characterized by its role in maintaining the integrity of temperature-sensitive biological products. These products, which include vaccines, blood plasma, biologics, and certain pharmaceuticals, require highly controlled storage conditions, either refrigerated or frozen, to ensure their efficacy and safety. With increasing demand for such products, driven by the global rise in biopharmaceutical manufacturing, the GMP storage market is witnessing rapid expansion.
The GMP storage market is an integral part of the global cold chain market, accounting for around 4.7% of the total cold chain market, which was valued at approximately US$ 119.98 billion in 2021. This market's growth is being driven by several factors, including the surge in biologics production, regulatory demands, and the shift towards outsourced storage services. These factors have led to a steady increase in demand for high-quality storage solutions that comply with stringent GMP standards.
Persistence Market Research has identified key growth drivers and challenges in the market. While the sector is growing rapidly, it also faces challenges such as the high capital expenditure required for building and maintaining GMP storage facilities. Despite these challenges, the demand for temperature-controlled storage is expected to rise significantly in the coming years.
Key Highlights from the Report
• The GMP storage market size was valued at US$ 5.9 billion in 2022.
• The market is projected to reach US$ 10.2 billion by 2032, growing at a 5.6% CAGR.
• Temperature-sensitive biologics are the leading application segment, accounting for 38% of the market share in 2021.
• North America dominates the GMP storage market, holding 69% of the total market share in 2021.
• The market for outsource GMP storage services is growing rapidly, driven by demand from biopharmaceutical companies.
• Technological innovations in cold chain storage equipment, such as low-energy biomedical freezers, are driving market expansion.
Market Segmentation
The GMP storage market can be segmented into various categories, including product type, end-users, and regional markets. These segments are critical for understanding the diverse applications and trends within the market.
Product Type: The market is primarily divided into refrigeration and freezing equipment used for maintaining the integrity of temperature-sensitive products. The biomedical refrigerators and freezers segment dominates the market, accounting for a significant share due to the increasing demand for temperature-controlled storage solutions in the pharmaceutical and biotechnology sectors.
End-Users: The demand for GMP storage products and services is largely driven by the biopharmaceutical sector. This sector, which includes companies involved in the development, manufacturing, and distribution of biologics, holds the largest share in the market. Biopharmaceutical companies often outsource GMP storage services to manage their storage needs more effectively, particularly when dealing with high-value or critical temperature-sensitive products.
Read Detailed Analysis: https://www.persistencemarketresearch.com/market-research/gmp-storage-market.asp
Regional Insights
The North American GMP storage market leads in terms of market share, with the United States accounting for more than 89% of the North American market in 2021. The U.S. is witnessing significant demand for GMP storage services due to the FDA's approval of temperature-sensitive drugs, particularly vaccines and biologics. The regulatory environment in the U.S. is robust, ensuring the adoption of the latest technologies and the highest standards in cold chain storage.
In Europe, Germany stands out as the largest market for GMP storage, accounting for around 29.7% of the market share in 2021. The biopharmaceutical industry in Germany continues to expand, driven by the growth of biotechnology companies and significant investments in research and development. This expansion has increased the need for high-quality GMP storage solutions to ensure product integrity.
China, with its rapidly growing biotechnology sector and government investment in biopharmaceutical research, has become an emerging market for GMP storage. The Chinese government has been investing heavily in biotech research and infrastructure, making East Asia a key region for GMP storage services.
Market Drivers
Several factors are driving the growth of the GMP storage market:
Surge in Biopharmaceutical Production: The increasing production of biologics, such as vaccines, gene therapies, and monoclonal antibodies, requires stringent temperature control, thus boosting demand for GMP storage.
Regulatory Compliance: Stringent regulations around the storage and transportation of pharmaceutical products are forcing companies to adopt GMP-compliant storage solutions. Regulatory bodies, including the FDA, have raised the standards for storage conditions, particularly for temperature-sensitive drugs.
Technological Advancements: Innovations in cold chain technologies, such as low-energy biomedical refrigerators and controlled-rate freezers, are contributing to the market's growth by providing more efficient and cost-effective storage solutions.
Outsourcing Storage Services: Many pharmaceutical companies are outsourcing GMP storage services to manage costs and scalability. This trend is particularly beneficial for smaller companies that may not have the resources to build and maintain in-house GMP storage facilities.
Market Restraints
Despite the growth prospects, the GMP storage market faces several challenges:
High Capital Investment: The establishment of GMP-compliant storage facilities involves substantial capital expenditure. This includes investments in refrigeration equipment, security systems, inventory management systems, and backup power solutions.
Energy Costs: Maintaining temperature-controlled storage facilities requires significant energy consumption. In regions where electricity costs are high, this can make GMP storage operations expensive for providers and manufacturers.
Complex Regulatory Compliance: Adhering to ever-evolving regulatory requirements across multiple jurisdictions can be challenging for companies operating globally. The complexity of maintaining compliance with various local and international standards can create barriers to entry for new players in the market.
Market Opportunities
The GMP storage market also presents several opportunities:
Expansion of Biotech Industry: With governments investing heavily in biotechnology and life sciences, particularly in countries like China and the U.S., the demand for GMP storage services is set to increase.
Emerging Markets: Developing countries are showing an increased demand for cold chain solutions, driven by the rising healthcare needs of their populations. This presents significant opportunities for GMP storage providers to expand into new regions.
Technological Innovation: The introduction of more energy-efficient, cost-effective, and reliable storage equipment presents opportunities for companies to differentiate themselves and expand their market share.
Request for Customization of the Research Report: https://www.persistencemarketresearch.com/request-customization/33113
Reasons to Buy the Report
• Gain a comprehensive understanding of the GMP storage market and its dynamics.
• Identify key growth drivers and market challenges to make informed business decisions.
• Stay ahead of technological trends and innovations shaping the market.
• Understand the competitive landscape and strategies of key players.
• Leverage regional insights to tap into high-growth markets.
Frequently Asked Questions (FAQs)
• How big is the GMP storage market?
• Who are the key players in the global GMP storage market?
• What is the projected growth rate of the GMP storage market?
• What is the market forecast for the GMP storage market by 2032?
• Which region is estimated to dominate the GMP storage market through the forecast period?
Company Insights
Key players in the GMP storage market include:
• ThermoGenesis Holdings, Inc.
• ThermoFisher Scientific Inc.
• BioLife Solutions, Inc.
• Danaher (Cytiva)
• MEDIPOST
• REMI GROUP
• Eurofins Scientific
• Eppendorf
• Intertek Group Plc
• Arctiko
• Bioline Technologies
• Hindustan Apparatus Mfg. Co.
• SY-LAB Geräte GmbH
• BioConvergence LLC
• PHC Holdings Corporation
Recent Developments:
BioLife Solutions, Inc. acquired Global Cooling, Inc. in May 2021, expanding its portfolio of temperature-controlled storage solutions.
BioLife Solutions, Inc. launched a new high-capacity controlled-rate freezer line in April 2021, marking a significant step in improving cold chain storage for biologics.
The GMP storage market is poised for significant growth over the next decade, driven by increasing demand for biologics, technological advancements, and the rising trend of outsourcing storage services. The strategic actions of leading players and regional trends will continue to shape the market landscape as new opportunities and challenges emerge.
Read More Related Reports:
Mri Guided Focused Ultrasound Devices Market https://www.persistencemarketresearch.com/market-research/mri-guided-focused-ultrasound-devices-market.asp
Sports Medicine Market https://www.persistencemarketresearch.com/market-research/sports-medicine-market.asp
Cryotherapy Market https://www.persistencemarketresearch.com/market-research/cryotherapy-market.asp
Vein Illuminator Market https://www.persistencemarketresearch.com/market-research/vein-illuminator-market.asp
Oligonucleotide Therapeutics Market https://www.persistencemarketresearch.com/market-research/oligonucleotide-therapeutics-market.asp
Contact Us:
Persistence Market Research
G04 Golden Mile House, Clayponds Lane
Brentford, London, TW8 0GU UK
USA Phone: +1 646-878-6329
UK Phone: +44 203-837-5656
Email: sales@persistencemarketresearch.com
Web: https://www.persistencemarketresearch.com
About Persistence Market Research:
At Persistence Market Research, we specialize in creating research studies that serve as strategic tools for driving business growth. Established as a proprietary firm in 2012, we have evolved into a registered company in England and Wales in 2023 under the name Persistence Research & Consultancy Services Ltd. With a solid foundation, we have completed over 3600 custom and syndicate market research projects, and delivered more than 2700 projects for other leading market research companies' clients.
Our approach combines traditional market research methods with modern tools to offer comprehensive research solutions. With a decade of experience, we pride ourselves on deriving actionable insights from data to help businesses stay ahead of the competition. Our client base spans multinational corporations, leading consulting firms, investment funds, and government departments. A significant portion of our sales comes from repeat clients, a testament to the value and trust we've built over the years.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release GMP Storage Market to See Steady Gains with Rising Drug Manufacturing Activities - Persistence Market Research here
News-ID: 4180198 • Views: …
More Releases from Persistence Market Research
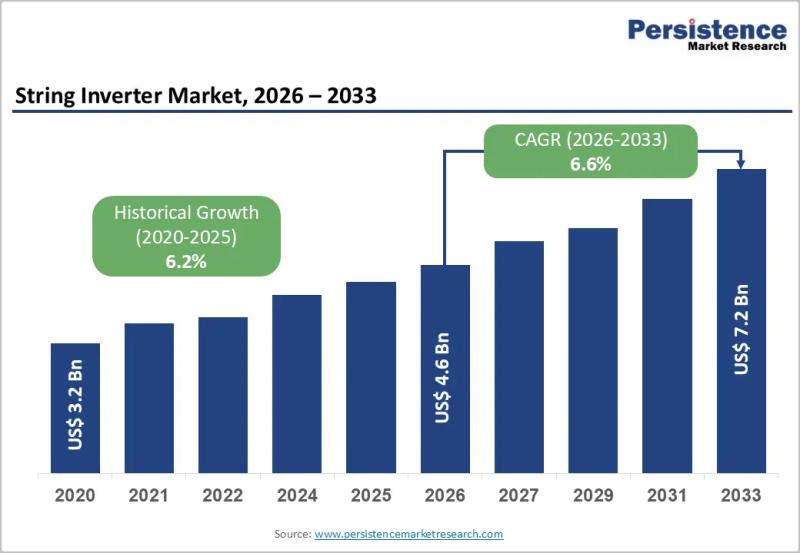
String Inverter Market to Reach US$7.2 Billion by 2033 - Persistence Market Rese …
The string inverter market is emerging as a critical pillar of the global solar photovoltaic ecosystem, supporting the transition toward decentralized renewable energy systems. String inverters play a central role in converting direct current generated by solar panels into usable alternating current for grid connection or onsite consumption. Compared to central inverters, string inverters provide greater design flexibility, easier installation, and improved system monitoring at the string level. Their widespread…
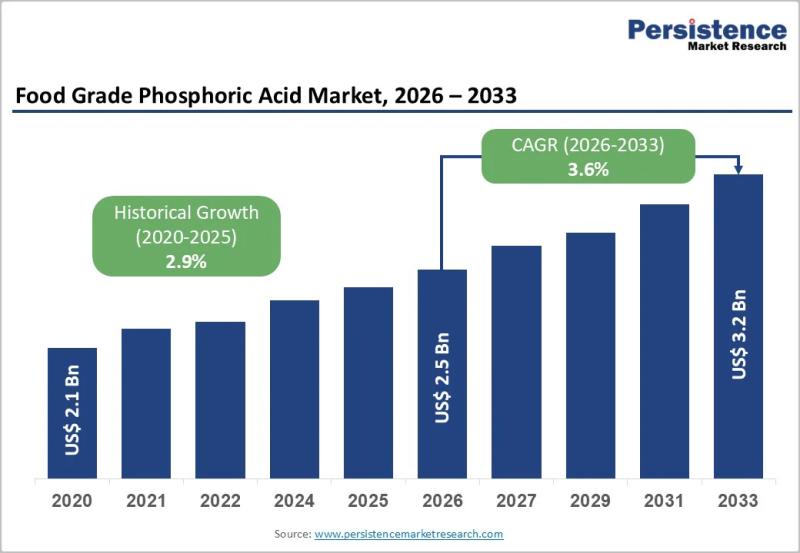
Food Grade Phosphoric Acid Market to Reach US$3.2 Billion by 2033 - Persistence …
The food grade phosphoric acid market plays a critical role in the global food and beverage industry, serving as a key ingredient in acidity regulation, flavor enhancement, preservation, and pH stabilization. Food grade phosphoric acid is widely used in carbonated soft drinks, processed foods, dairy products, bakery formulations, and meat processing applications. Its ability to provide a sharp tangy taste and extend shelf life makes it indispensable for beverage manufacturers…
Electronic Document Management System (EDMS) Market to Surpass US$ 27.7 Billion …
The global Electronic Document Management System (EDMS) Market is projected to be valued at US$ 9.7 billion in 2026 and is expected to reach US$ 27.7 billion by 2033, expanding at a robust CAGR of 16.2% during 2026-2033. Organizations worldwide are accelerating digital transformation initiatives by shifting from paper-based processes to intelligent document management platforms. This structural transition is enabling enterprises to enhance workflow automation, improve data governance, and reduce…

Intelligent Automation Market to Reach US$ 25.8 Billion by 2033 Driven by AI, Cl …
The global Intelligent Automation Market is projected to be valued at US$ 12.6 billion in 2026 and is forecast to reach US$ 25.8 billion by 2033, expanding at a CAGR of 10.8% during 2026-2033. Growth is primarily driven by rapid advancements in artificial intelligence (AI), machine learning (ML), robotic process automation (RPA), and cloud computing technologies. Businesses across sectors are increasingly integrating intelligent automation solutions to enhance operational efficiency, reduce…
More Releases for GMP
Creative Peptides Released GMP Synthesis Service
Located in Shirley, New York, the world’s leading peptide supplier Creative Peptides announced the launch of its GMP synthesis (https://www.creative-peptides.com/services/custom-gmp-peptide-synthesis-services.html ) business on August 29, 2018. Now this company is focused on the development and GMP manufacturing of pharmaceutical grade peptides.
As the demand of pharmaceutical market continues to grow, more and more pharmas and research institutions choose the CMO and CRO models to expand their businesses, which is more…
Diapharm implements European GMP guidelines in China
Münster (DE), London (UK), Ningbo (CN), 20 December 2013 – Pharmaceutical service provider Diapharm (diapharm.com) is increasing its business activities in China: Diapharm has now implemented a “European” quality management system for Neptune Pharma Ltd (www.neptunepharma.com) in their Joint Venture Partner’s factory in Ningbo, Zhejiang Province. And it has done so successfully: The veterinary medicinal product Trident 500mg/g Powder for Suspension for Fish Treatment (www.trident-50.com), is manufactured onsite under EU…
ECA Foundation releases free GMP WebApp
The ECA Foundation has been providing advanced training and information services in the pharmaceutical industry and especially with regard to pharmaceutical Quality Assurance and GMP compliance for more than 10 years. Now the organisation took advantage of its extensive experience to develop a further free of charge service – the new GMP WebApp.
This new GMP WebApp runs on all smartphones and tablet PCs (Apple and Android platforms) and allows users…
GMP Friction Products Awarded ISO 9001:2008
Internationally Recognized Certification Measures Consistency in Process, Procedure and Quality Performance in Manufacture of Friction Materials
AKRON, OH (March 23, 2011) -- GMP Friction Products, a world leader manufacturing powdered metal friction products for clutch plates and brake pads, recently received certification for ISO 9001:2008.
“ISO 9001:2008 signifies we have taken the extra measure of documenting the policies and standards to ensure consistent compliance with our manufacturing processes,” said Jerry Lynch,…
GMP MANUAL Volume 2 - Validation Procedures by Maas & Peither AG – GMP Publish …
GMP Publishing is launching its new GMP MANUAL Volume 2 – Validation Procedures.
The compendium on validation procedures was written by Dr. Doris Borchert, Dr. Peter Bosshard, Dr. Ralph Gomez, Dr. Michael Hiob, Dr. Christine Oechslein, Max Lazar, Ulrike Reuter, Michael Schulte, Uwe Schwarzat – all international experts and key opinion leaders. They share their detailed understanding of the various aspects of the validation process in clear and comprehensive style…
blue inspection body celebrates 50 GMP audits
Münster (Germany), 20 November 2009. Two years after founding the company and just 18 months after gaining the accreditation blue inspection body GmbH announced today the successful execution of its 50th GMP audit. Further audit trips to China, India, Israel and various European countries have been scheduled already, meaning that in the first quarter 2010 the 75th audit is targeted to be completed. Blue, as a privately organised inspection body,…
