Press release
North America Duchenne muscular dystrophy (DMD) treatment market Size to Reach US$ 8.84 billion by 2033 - Exclusive Report by DataM Intelligence
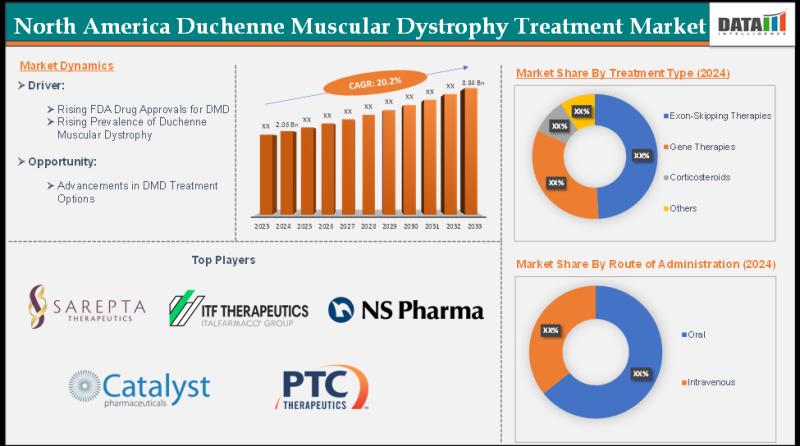
The North America Duchenne muscular dystrophy (DMD) treatment market was valued at US$ 2.06 billion in 2024 and is projected to reach US$ 8.84 billion by 2033, expanding at a CAGR of 20.2% during the forecast period 2025-2033.Download your exclusive sample report today: (corporate email gets priority access): https://www.datamintelligence.com/download-sample/north-america-duchenne-muscular-dystrophy-treatment-market?sg
Market Drivers:
➠ Rising Prevalence of DMD
The growing number of diagnosed cases and improved genetic screening are expanding the patient pool, fueling demand for effective therapies.
➠ Advancements in Gene and Cell Therapies
Breakthroughs in exon-skipping drugs, CRISPR-based research, and gene replacement therapies are accelerating treatment adoption in the region.
➠ Strong Regulatory Framework and Orphan Drug Incentives
U.S. FDA designations such as Fast Track, Breakthrough Therapy, and Orphan Drug status provide pharmaceutical companies with accelerated pathways, encouraging innovation.
➠ High Healthcare Spending and Insurance Coverage
Robust healthcare expenditure, coupled with reimbursement for high-cost therapies, makes North America a favorable market for novel DMD treatments.
➠ Active Research Collaborations and Clinical Trials
Academic institutions, biotech firms, and patient advocacy groups play a key role in driving clinical research and expanding treatment accessibility across the region.
Looking For Full Report? Get it Here: https://www.datamintelligence.com/buy-now-page?report=north-america-duchenne-muscular-dystrophy-treatment-market
Market Segments:
By Treatment Type (Exon-Skipping Therapies, Gene Therapies, Corticosteroids, Others)
By Route of Administration (Oral, Intravenous)
Latest clinical / regulatory developments (2025):
✅ Avidity's del-zota (delpacibart zotadirsen) - FDA Breakthrough Therapy designation (July 2025)
Avidity's exon-44-skipping AOC candidate showed statistically significant increases in exon skipping and dystrophin in EXPLORE44 (Phase 1/2); the FDA granted Breakthrough Therapy designation and Avidity is preparing materials for an accelerated approval filing targeted by year-end 2025.
✅ Dyne Therapeutics' DYNE-251 - FDA Breakthrough Therapy designation (Aug 4, 2025)
Dyne announced Breakthrough Therapy designation for DYNE-251 based on encouraging DELIVER study results showing sustained functional improvements.
Mergers, acquisitions, partnerships and financing activity 2025
✅ Novartis reportedly considering acquisition of Avidity (news reports, Aug 6, 2025 - exploratory stage)
Media coverage in Aug 2025 reported that Novartis was weighing a takeover of Avidity (Avidity's del-zota and other muscle-disease programs make it an attractive target); this was reported as preliminary/rumored.
✅ Sarepta ↔ Arrowhead commercial / financing actions (2025)
Sarepta and Arrowhead have had major commercial/strategic transactions in the past year (large licensing/milestone deals and Sarepta's later sales of Arrowhead stake to shore up cash were reported in 2025). These moves affect balance sheets and strategic focus for companies operating in the DMD / RNA therapeutics space.
Stay informed with the latest industry insights-start your subscription now: https://www.datamintelligence.com/reports-subscription?sg
Product launches / near-launch candidates & regulatory plans (2025):
✅ Del-zota (Avidity) - on track for regulatory filing / potential U.S. launch (BLA materials planned for submission by end-2025)
Breakthrough designation and positive EXPLORE44 data put del-zota in the "near-launch" category if FDA accelerated approval pathways progress favorably.
✅ DYNE-251 (Dyne Therapeutics) - Breakthrough designation (accelerated development path)
Breakthrough designation moves DYNE-251 into a prioritized regulatory pathway; not yet an approved launch but a candidate with potentially accelerated review.
✅ Elevidys (Sarepta) - previously approved gene therapy but regulatory status in flux in 2025
Elevidys remains the only gene therapy previously approved for some DMD patients; however, 2025 brought intense regulatory and safety scrutiny that affected availability and commercialization plans. (Not a 2025 new launch, but a major market product whose availability changed in 2025).
Market Key Players:
Key players are Sarepta Therapeutics, Inc., ITF Therapeutics LLC, NS Pharma, Inc., Catalyst Pharmaceuticals, Inc., and PTC Therapeutics. Emerging players in the market include F. Hoffmann-La Roche Ltd, Capricor Therapeutics, Inc., REGENXBIO Inc., Solid Biosciences Inc., Wave Life Sciences and Genethon.
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release North America Duchenne muscular dystrophy (DMD) treatment market Size to Reach US$ 8.84 billion by 2033 - Exclusive Report by DataM Intelligence here
News-ID: 4175964 • Views: …
More Releases from DataM Intelligence 4 Market Research LLP
Non-Opioid Pain Treatment Market to Reach USD 9.2 Billion by 2033 at 8.1% CAGR | …
The Non-Opioid Pain Treatment Market was valued at approximately USD 4.6 billion in 2024 and is expected to reach around USD 9.2 billion by 2033, growing at a CAGR of about 8.1% during the forecast period from 2025 to 2033. Market growth is primarily driven by the global effort to reduce opioid dependence, rising prevalence of chronic pain conditions such as arthritis, neuropathic pain, and musculoskeletal disorders, and increasing regulatory…

United States Food Waste Management Market 2026 | Market with Growth Drivers and …
Market Size and Growth
Food Waste Management Market reached US$69.8 billion in 2024 and is expected to reach US$136.2 billion by 2032, growing with a CAGR of 8.7% during the forecast period 2025-2032
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://datamintelligence.com/download-sample/food-waste-management-market?kb
Food waste management isn't just about cleaning up; it's a dynamic industry transforming scraps into energy, fertilizers, and even profits. In this article, we'll…

Information Security Consulting Market Set for Explosive Growth to US$ 39.6 Bill …
The Global Information Security Consulting Market reached USD 24.1 billion in 2022 and is expected to reach USD 39.6 billion by 2030, growing with a CAGR of 10.7% during the forecast period 2024-2031.
Market growth is driven by the escalating frequency and sophistication of cyberattacks, stringent regulatory compliance requirements like GDPR and CCPA, and the rapid adoption of cloud computing, IoT, and AI technologies. Advancements in AI-driven threat detection, rising demand…

Bivalvia Market Forecast for Robust Growth to USD 25,602.6 million by 2030, Anch …
Market Overview
The Bivalvia Market size reached USD 19,899.7 million in 2022 and is projected to witness lucrative growth by reaching up to USD 25,602.6 million by 2030. The market is growing at a CAGR of 3.2% during the forecast period (2024-2031).
The Bivalvia market is expanding due to rising global seafood consumption, increasing demand for protein-rich diets, growth in aquaculture production, improved cold-chain logistics, and strong export trade. Sustainability initiatives and…
More Releases for DMD
Duchenne Muscular Dystrophy (DMD) Market Growth in 2034
Market Overview
The Duchenne Muscular Dystrophy (DMD) Market is expanding rapidly as advances in genetic medicine, exon-skipping therapies, gene therapy platforms, and improved diagnostic capabilities reshape treatment options for this severe, progressive neuromuscular disorder.
DMD is caused by mutations in the dystrophin gene, leading to muscle degeneration beginning in early childhood. Growing awareness among clinicians and caregivers, widespread adoption of next-generation sequencing (NGS), and increasing availability of disease-modifying therapies have significantly strengthened…
Emerging Trends to Reshape the Duchenne Muscular Dystrophy (DMD) Therapeutics Ma …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
Duchenne Muscular Dystrophy (DMD) Therapeutics Market Size Valuation Forecast: What Will the Market Be Worth by 2025?
The market size for duchenne muscular dystrophy (DMD) therapeutics has seen a significant increase in the recent years. The market, which was valued at $11.95 billion in 2024, is anticipated to expand…
Emerging Trends Influencing The Growth Of The Duchenne Muscular Dystrophy (DMD) …
The Duchenne Muscular Dystrophy (DMD) Therapeutics Market Report by The Business Research Company delivers a detailed market assessment, covering size projections from 2025 to 2034. This report explores crucial market trends, major drivers and market segmentation by [key segment categories].
How Big Is the Duchenne Muscular Dystrophy (DMD) Therapeutics Market Size Expected to Be by 2034?
In recent times, the market size for Duchenne Muscular Dystrophy (DMD) therapeutics has seen a significant…
Leading Portland Dentist Michael P. Naughton, DMD, Unveils Magnolia Dental
Image: https://www.globalnewslines.com/uploads/2025/03/1741300210.jpg
Dr. Naughton is sharing the official new name of his renowned dental office, which provides premier dental services for the Portland, Oregon, region.
PORTLAND, OREGON - March 7th, 2025 - Michael P. Naughton, DMD, is excited to announce the official new name of his dental office. New and existing clients alike are now being greeted by Magnolia Dental [https://magnoliadentalpdx.com/], which offers the same office, staff, and exceptional level of dental…
Shaping the Duchenne Muscular Dystrophy (DMD) Therapeutics Market in 2025: Bit B …
How Big Is the Duchenne Muscular Dystrophy (DMD) Therapeutics Market Expected to Be, and What Will Its Growth Rate Be?
The Duchenne muscular dystrophy (DMD) therapeutics market will grow from $11.95 billion in 2024 to $16.45 billion in 2025, at a CAGR of 37.6%. The growth is attributed to the increasing prevalence of Duchenne muscular dystrophy, rising awareness of treatment options, healthcare spending, and government initiatives.
The Duchenne muscular dystrophy (DMD) therapeutics…
Hershey Family Dentistry Rebrands Under Dr. William Svitko, DMD
Award nominee Dr. William Svitko has become a widely recognized and sought after dentist in the Hershey, PA, area.
Image: https://www.getnews.info/uploads/ca064454a8c76f46c800ec30173f7d6d.png
Progressive Dental Concepts (PDC) is excited to announce its rebranding of Wesley R Davis Family Dentistry to Hershey Family Dentistry (HFD), marking a new chapter in the practice's history under the leadership of Dr. William Svitko, DMD. The rebrand includes the launch of a new, user-friendly website, designed to enhance patient…