Press release
Polypoidal choroidal vasculopathy Clinical Trials Assessment, 2025 by DelveInsight | Avirmax Biopharma, Novartis
DelveInsight's, "Polypoidal choroidal vasculopathy - Pipeline Insight, 2025" report provides comprehensive insights about 3+ companies and 3+ pipeline drugs in Polypoidal choroidal vasculopathy pipeline and Polypoidal choroidal vasculopathype. It covers the pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type.DelveInsight's Polypoidal choroidal vasculopathy Clinical Trials Analysis Report provides deep insights to support strategic decision-making for Polypoidal choroidal vasculopathy pipeline expansion, competitive positioning, and partnership identification in the rare neurodegenerative disease space.
Polypoidal choroidal vasculopathy Overview:
Polypoidal choroidal vasculopathy (PCV) is a choroidal eye disorder characterized by abnormal, polyp-like blood vessels that can lead to significant vision loss. Common symptoms include sudden blurred vision, central blind spots (scotomas), and distorted vision (metamorphopsia), which often remain consistent throughout the day. The disease is associated with serosanguineous detachments of the retinal pigment epithelium and is believed to stem from abnormalities in the inner choroidal vessels. While the exact cause is still unclear, both genetic predisposition and environmental factors are thought to play a role. PCV also shares clinical features with wet age-related macular degeneration, suggesting overlapping underlying mechanisms.
Download our report @ https://www.delveinsight.com/report-store/polypoidal-choroidal-vasculopathy-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr
"Polypoidal choroidal vasculopathy Pipeline Insight 2025" report by DelveInsight provides a comprehensive analysis of the ongoing clinical development activities and growth prospects across the Polypoidal choroidal vasculopathy Therapeutics Market.
Key Takeaways from the Polypoidal choroidal vasculopathy Pipeline Report
*
DelveInsight's Polypoidal Choroidal Vasculopathy (PCV) Pipeline Report highlights an active landscape with over 3 companies developing more than 3 therapeutic candidates for PCV.
*
In May 2024, Avirmax Biopharma received Investigational New Drug (IND) clearance from the U.S. FDA to launch a Phase I/IIa trial of its gene therapy candidate, ABI-110. This treatment utilizes an engineered adeno-associated virus capsid, AAV2.N54, to deliver a therapeutic transgene directly to the macular retina via intravitreal injection. The approach is designed to target the genetic drivers of PCV while potentially reducing the need for frequent injections, which remain the current standard of care.
*
Leading players in the PCV space include Avirmax Biopharma, Novartis, and others, all working to advance novel therapies. Among the most promising candidates is ABI-110, alongside additional pipeline assets aiming to transform the treatment landscape.
Polypoidal choroidal vasculopathy Pipeline Analysis
The Polypoidal choroidal vasculopathy pipeline insights report 2025, provides insights into:
*
Provides comprehensive insights into key companies developing therapies in the Polypoidal choroidal vasculopathy Market.
*
Categorizes Polypoidal choroidal vasculopathy therapeutic companies by development stage: early, mid, and late-stage.
*
Highlights major companies involved in targeted therapy development, including both active and inactive (paused/discontinued) projects.
*
Reviews emerging Polypoidal choroidal vasculopathy drugs under development based on:
*
Stage of development
*
Polypoidal choroidal vasculopathy Route of administration
*
Target receptor
*
Monotherapy vs. combination therapy
*
Polypoidal choroidal vasculopathy Mechanism of action
*
Molecular type
*
Offers detailed analysis of:
*
Company-to-company and company-academia collaborations
*
Polypoidal choroidal vasculopathy Licensing agreements
*
Funding and investment activities supporting future Polypoidal choroidal vasculopathy market advancement.
Request for a sample report @ https://www.delveinsight.com/sample-request/polypoidal-choroidal-vasculopathy-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr
Polypoidal choroidal vasculopathy Emerging Drugs
*
ABI-110: Avirmax Biopharma
ABI-110, developed by Avirmax Biopharma, is an advanced gene therapy currently under evaluation in Phase I/IIa clinical trials for wet age-related macular degeneration (AMD) and polypoidal choroidal vasculopathy (PCV). The therapy employs a specially engineered adeno-associated virus capsid, AAV2.N54, to deliver a therapeutic gene directly to the macular retina, aiming to correct underlying genetic drivers rather than simply alleviate symptoms. A dedicated Phase I/II trial is also underway to assess its potential specifically in PCV patients.
Polypoidal choroidal vasculopathy Companies
More than three key companies are developing therapies for polypoidal choroidal vasculopathy (PCV), with Avirmax Biopharma at the forefront, advancing a candidate currently in Phase I/II trials, the most progressed stage of development.
DelveInsight's report covers around 3+ products under different phases of Polypoidal choroidal vasculopathy clinical trials like
*
Polypoidal choroidal vasculopathy Late stage Therapies (Phase III)
*
Polypoidal choroidal vasculopathy Mid-stage Therapies (Phase II)
*
Polypoidal choroidal vasculopathy Early-stage Therapies (Phase I)
*
Polypoidal choroidal vasculopathy Pre-clinical and Polypoidal choroidal vasculopathy Discovery stage Therapies
*
Polypoidal choroidal vasculopathy Discontinued & Inactive Therapies
Polypoidal choroidal vasculopathy pipeline report provides the Polypoidal choroidal vasculopathy therapeutic assessment of the pipeline drugs by the Route of Administration. Products have been categorized under various ROAs such as
*
Intravenous
*
Subcutaneous
*
Oral
*
Intramuscular
Polypoidal choroidal vasculopathy Products have been categorized under various Molecule types such as
*
Monoclonal antibody
*
Small molecule
*
Peptide
Download Sample Pages to Get an in-depth Assessment of the Emerging Polypoidal choroidal vasculopathy Therapies and Key Polypoidal choroidal vasculopathy Companies: Polypoidal choroidal vasculopathy Clinical Trials and recent advancements [https://www.delveinsight.com/report-store/polypoidal-choroidal-vasculopathy-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
Major trends shaping the clinical trial and competitive landscape for Polypoidal choroidal vasculopathy:
*
Shift Toward Gene and Targeted Therapies: Development of gene therapies and novel biologics aimed at addressing underlying disease mechanisms rather than just managing symptoms.
*
Expansion of Clinical Trials: Increased activity in early- and mid-stage trials, including multinational studies to improve patient diversity and data robustness.
*
Emerging Biomarkers and Diagnostics: Use of imaging techniques and molecular biomarkers to improve patient selection, monitor disease progression, and optimize endpoints.
*
Regulatory Incentives: Orphan Drug, Fast Track, and RMAT designations encourage rapid development and approval for rare ocular diseases.
*
Strategic Collaborations: Partnerships among biotech, pharmaceutical companies, and academic institutions to share expertise and accelerate development.
*
Innovation in Drug Delivery: Focus on intravitreal injections, engineered viral vectors, and long-acting formulations to improve efficacy and reduce treatment burden.
*
Competitive Differentiation: Companies positioning pipelines based on mechanism of action, molecular type, and therapeutic approach.
*
Patient-Centric Outcomes: Incorporation of vision-related quality-of-life measures and functional endpoints in clinical trials.
*
Integration of Real-World Evidence: Using real-world data to support regulatory submissions and reimbursement strategies.
Polypoidal choroidal vasculopathy Pipeline Therapeutic Assessment
- Polypoidal choroidal vasculopathy Assessment by Product Type
- Polypoidal choroidal vasculopathy By Stage
- Polypoidal choroidal vasculopathy Assessment by Route of Administration
- Polypoidal choroidal vasculopathy Assessment by Molecule Type
Polypoidal choroidal vasculopathy Report Section Includes:
*
Polypoidal choroidal vasculopathy competitive landscape clinical trials
*
Polypoidal choroidal vasculopathy emerging drugs analysis
*
Polypoidal choroidal vasculopathy investigational drugs report
*
Polypoidal choroidal vasculopathy clinical trial trends analysis
*
Polypoidal choroidal vasculopathy pipeline benchmarking report
*
Polypoidal choroidal vasculopathy drug pipeline competitive intelligence
*
Polypoidal choroidal vasculopathy competitive landscape
Download Polypoidal choroidal vasculopathy Sample report to know in detail about the Polypoidal choroidal vasculopathy treatment market @ Polypoidal choroidal vasculopathy Therapeutic Assessment [https://www.delveinsight.com/sample-request/polypoidal-choroidal-vasculopathy-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
Table of Content
1. Report Introduction
2. Executive Summary
3. Polypoidal choroidal vasculopathy Current Treatment Patterns
4. Polypoidal choroidal vasculopathy - DelveInsight's Analytical Perspective
5. Therapeutic Assessment
6. Polypoidal choroidal vasculopathy Late-Stage Products (Phase-III)
7. Polypoidal choroidal vasculopathy Mid-Stage Products (Phase-II)
8. Early Stage Products (Phase-I)
9. Pre-clinical Products and Discovery Stage Products
10. Inactive Products
11. Dormant Products
12. Polypoidal choroidal vasculopathy Discontinued Products
13. Polypoidal choroidal vasculopathy Product Profiles
14. Polypoidal choroidal vasculopathy Key Companies
15. Polypoidal choroidal vasculopathy Key Products
16. Dormant and Discontinued Products
17. Polypoidal choroidal vasculopathy Unmet Needs
18. Polypoidal choroidal vasculopathy Future Perspectives
19. Polypoidal choroidal vasculopathy Analyst Review
20. Appendix
21. Report Methodology
Request the Sample PDF to Get Detailed Insights About the Polypoidal choroidal vasculopathy Pipeline Reports Offerings [https://www.delveinsight.com/report-store/polypoidal-choroidal-vasculopathy-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports Pharma companies by providing comprehensive end-to-end solutions to improve their performance.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Kritika Rehani
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=polypoidal-choroidal-vasculopathy-clinical-trials-assessment-2025-by-delveinsight-avirmax-biopharma-novartis]
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: Nevada
Country: United States
Website: https://www.delveinsight.com/
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Polypoidal choroidal vasculopathy Clinical Trials Assessment, 2025 by DelveInsight | Avirmax Biopharma, Novartis here
News-ID: 4174559 • Views: …
More Releases from ABNewswire
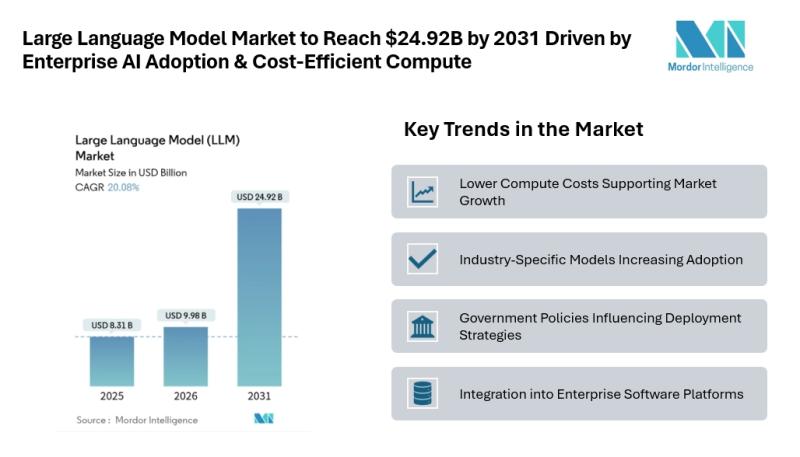
Large Language Model Market to Reach $24.92B by 2031 Driven by Enterprise AI Ado …
Mordor Intelligence has published a new report on the large language model market, offering a comprehensive analysis of trends, growth drivers, and future projections.
Large Language Model Market Outlook
According to Mordor Intelligence, the LLM market size [https://www.mordorintelligence.com/industry-reports/large-language-model-llm-market?utm_source=abnewswire] was valued at USD 8.31 billion in 2025 and is estimated to grow to USD 9.98 billion in 2026, reaching USD 24.92 billion by 2031 at a CAGR of 20.08% during the forecast period.…

Self Employed Tax Software UK: Why Freelancers and Sole Traders Are Switching to …
With Many individuals are seeking software that simplifies tax filing while ensuring full compliance with HMRC requirements. Manual spreadsheets and paper-based calculations are being replaced by real-time, automated systems that give users visibility over their tax position throughout the year. Among the platforms gaining traction is Pie, a UK-based digital tax app built specifically to support self-employed individuals with modern income needs.
LONDON, United Kingdom - February 19, 2026 - Demand…
CivicMail.org Reinvents Postcard Campaigns for Grassroots Advocacy
CivicMail.org aims to bring civic engagement back to basics through the power of pen, paper, and postage.
Image: https://www.abnewswire.com/upload/2026/02/2addd1e9e0381d7e2262e1edbb064123.jpg
CivicMail.org [https://civicmail.org/] has announced its launch to help Americans send real, physical postcards to their elected officials with just a few clicks, delivering personalized messages directly to the desks of decision-makers at the local, state, and federal levels.
Research shows [https://www.concordia.ca/news/stories/2021/09/24/personalized-messages-are-more-likely-to-get-a-response-from-politicians-new-research-finds.html] that physical mail carries more weight with elected officials than petitions, emails, or…

New Children's Story: The Story of Sharin' Bear
A Heartfelt Message Of Courage, Kindness, And The True Meaning Of Giving
A pleasant new story for children, The Story of Sharin' Bear by Sharon Woods , introduces families to a lovable little cub whose journey of bravery and compassion changes him into a representation of sharing for children globally.
Entrenched in adventure, innocence, and emotional growth, this uplifting tale offers an unforgettable reminder that even the smallest acts of kindness can…
More Releases for Polypoidal
Centronuclear Myopathy Market Projected to Grow at 7.8% CAGR
Introduction
Centronuclear myopathy (CNM) is a rare genetic neuromuscular disorder characterized by abnormal central positioning of nuclei in skeletal muscle fibers, leading to muscle weakness, hypotonia, delayed motor milestones, and in severe cases, respiratory complications. It includes subtypes such as X-linked myotubular myopathy (XLMTM), autosomal recessive CNM, and autosomal dominant CNM.
Download Full PDF Sample Copy of Market Report @ https://exactitudeconsultancy.com/request-sample/72249
Although treatment is largely supportive today, breakthroughs in gene therapies, enzyme replacement…
Vagus Nerve Stimulator Market to Grow at 6.8% CAGR, Reaching USD 1.25 Billion by …
Introduction
Vagus nerve stimulation (VNS) is a neuromodulation therapy that uses electrical impulses delivered to the vagus nerve to regulate brain activity. Initially approved for drug-resistant epilepsy, VNS has since expanded into treatments for depression, migraine, cluster headaches, and inflammatory conditions.
With rising cases of neurological and psychiatric disorders, the growing preference for non-pharmacological therapies, and ongoing innovation in implantable and non-invasive devices, the vagus nerve stimulator market is positioned for significant…
Polypoidal choroidal vasculopathy Pipeline Analysis and Clinical Trials Assessme …
Polypoidal choroidal vasculopathy Pipeline constitutes 3+ key companies continuously working towards developing 3+ Polypoidal choroidal vasculopathy treatment therapies, analyzes DelveInsight.
Polypoidal choroidal vasculopathy Overview:
Polypoidal choroidal vasculopathy (PCV) is an eye disease affecting the choroid, marked by abnormal, polyp-like blood vessels that can cause serious vision problems. It often presents with sudden blurred vision, central blind spots (scotomas), and distorted vision (metamorphopsia), typically remaining stable throughout the day. PCV leads to serosanguineous…
Polypoidal Choroidal Vasculopathy (PCV) Market to Grow at a Substantial Growth R …
DelveInsight's "Polypoidal Choroidal Vasculopathy Market Insights, Epidemiology, and Market Forecast 2032" report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the Polypoidal Choroidal Vasculopathy Market Size and Share in the seven major markets (7MM) (i.e., the United States, EU4 (Germany, Spain, Italy, France), the United Kingdom and Japan).
The market report covers emerging drugs, treatment practices, market share of individual Polypoidal Choroidal Vasculopathy therapies, and…
Cervical Dysplasia Pipeline Assessment Report (2023) Covering Clinical Trials, E …
Las Vega (Nevada), United States //- As per DelveInsight's assessment, globally, several major pharma and biotech companies are working on developing new therapies in the Cervical Dysplasia therapeutics landscape based on different Routes of Administration (ROA), Mechanisms of Action (MOA), and molecule types. Several of the therapies are in the advanced stages of clinical development and are expected to launch in the coming years.
"Cervical Dysplasia Pipeline Insight, 2023" report by…
Chlamydia Pipeline Assessment (2023 Updates) | In-depth Insights into the Clinic …
Las Vega (Nevada), United States //- As per DelveInsight's assessment, globally, about 5+ key pharma and biotech companies are working on 5+ pipeline drugs in the Chlamydia therapeutics landscape based on different Routes of Administration (ROA), Mechanism of Action (MOA), and molecule types. Several of the therapies are in the advanced stages of clinical development and are expected to launch in the coming years.
"Chlamydia Pipeline Insight, 2023" report by DelveInsight…
