Press release
X-linked retinitis pigmentosa Pipeline Analysis & Clinical Trials, 2025, DelveInsight | Biogen, 4 D Molecular therapeutics, Applied Genetics Technology Corporation
DelveInsight's, "X-linked Retinitis Pigmentosa - Pipeline Insight, 2025," report provides comprehensive insights about 5+ companies and 5+ pipeline drugs in X-linked Retinitis Pigmentosa pipeline landscape. It covers the pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.DelveInsight's X-linked retinitis pigmentosa Clinical Trials Analysis Report provides deep insights to support strategic decision-making for X-linked retinitis pigmentosa pipeline expansion, competitive positioning, and partnership identification in the rare neurodegenerative disease space.
X-linked retinitis pigmentosa Overview:
X-linked Retinitis Pigmentosa (XLRP) is a rare inherited genetic disorder caused by mutations on the X chromosome. It results in the gradual degeneration of retinal photoreceptor cells, leading to progressive loss of peripheral vision. The condition affects roughly 1 in 4,000 people worldwide. Being an X-linked recessive disorder, it primarily affects males, while females usually serve as carriers. Early signs include night blindness and a steadily narrowing visual field.
Download our report @ https://www.delveinsight.com/report-store/x-linked-retinitis-pigmentosa-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr
"X-linked retinitis pigmentosa Pipeline Insight 2025" report by DelveInsight provides a comprehensive analysis of the ongoing clinical development activities and growth prospects across the X-linked retinitis pigmentosa Therapeutics Market.
Key Takeaways from the X-linked retinitis pigmentosa Pipeline Report
*
DelveInsight's X-linked Retinitis Pigmentosa (XLRP) Pipeline Report highlights a dynamic space with over 5 companies developing more than 5 therapeutic candidates for XLRP.
*
In January 2025, Beacon Therapeutics received FDA RMAT designation for Laru-Zova, a gene therapy targeting XLRP. This status accelerates development and review processes, reflecting the therapy's potential to address unmet medical needs. Laru-Zova also holds Fast Track designation in the U.S., PRIME designation in the EU, and ILAP designation in the UK.
*
In April 2025, Atsena Therapeutics announced that the FDA granted RMAT designation to ATSN-201, a gene therapy for X-linked retinoschisis, a related retinal disorder, aiming to speed the development of treatments for serious conditions.
*
Bionic Sight's BS01 gene therapy also received RMAT designation in February 2025. Early Phase 1/2 trial results suggest potential benefits in restoring vision for patients with advanced retinitis pigmentosa.
*
Key players in the XLRP space include Biogen, 4D Molecular Therapeutics, Applied Genetics Technology Corporation, and others, all working to advance innovative treatments. Promising therapies in development include BIIB 112 (NSR-RPGR), 4D-125, and additional pipeline candidates aiming to transform the treatment landscape.
X-linked retinitis pigmentosa Pipeline Analysis
The X-linked retinitis pigmentosa pipeline insights report 2025, provides insights into:
*
Provides comprehensive insights into key companies developing therapies in the X-linked retinitis pigmentosa Market.
*
Categorizes X-linked retinitis pigmentosa therapeutic companies by development stage: early, mid, and late-stage.
*
Highlights major companies involved in targeted therapy development, including both active and inactive (paused/discontinued) projects.
*
Reviews emerging X-linked retinitis pigmentosa drugs under development based on:
*
Stage of development
*
X-linked retinitis pigmentosa Route of administration
*
Target receptor
*
Monotherapy vs. combination therapy
*
X-linked retinitis pigmentosa Mechanism of action
*
Molecular type
*
Offers detailed analysis of:
*
Company-to-company and company-academia collaborations
*
X-linked retinitis pigmentosa Licensing agreements
*
Funding and investment activities supporting future X-linked retinitis pigmentosa market advancement.
Request for a sample report @ https://www.delveinsight.com/sample-request/x-linked-retinitis-pigmentosa-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr
X-linked retinitis pigmentosa Emerging Drugs
*
BIIB 112 (NSR-RPGR): Biogen
Biogen's lead candidate, BIIB 112 (NSR-RPGR), is a gene therapy utilizing a standard AAV8 vector to deliver a codon-optimized RPGR gene. Currently in Phase II/III trials, it is being developed to treat rare X-linked Retinitis Pigmentosa and has received orphan drug designation. The ongoing study is evaluating the therapy's safety, tolerability, and its potential to stabilize and improve central vision.
*
4D-125: 4 D Molecular Therapeutics
4D Molecular Therapeutics' lead candidate, 4D-125, is being evaluated for safety and maximum tolerated dose in treating X-linked Retinitis Pigmentosa. This AAV-based gene therapy employs an optimized vector to deliver a functional RPGR gene directly to retinal photoreceptors.
X-linked retinitis pigmentosa Companies
More than five key companies are developing therapies for X-linked Retinitis Pigmentosa, with Biogen and others advancing candidates into mid-to-late-stage clinical trials (Phase II/III).
DelveInsight's report covers around 5+ products under different phases of X-linked retinitis pigmentosa clinical trials like
*
X-linked retinitis pigmentosa Late stage Therapies (Phase III)
*
X-linked retinitis pigmentosa Mid-stage Therapies (Phase II)
*
X-linked retinitis pigmentosa Early-stage Therapies (Phase I)
*
X-linked retinitis pigmentosa Pre-clinical and X-linked retinitis pigmentosa Discovery stage Therapies
*
X-linked retinitis pigmentosa Discontinued & Inactive Therapies
X-linked retinitis pigmentosa pipeline report provides the X-linked retinitis pigmentosa therapeutic assessment of the pipeline drugs by the Route of Administration. Products have been categorized under various ROAs such as
*
Intravenous
*
Subcutaneous
*
Oral
*
Intramuscular
X-linked retinitis pigmentosa Products have been categorized under various Molecule types such as
*
Monoclonal antibody
*
Small molecule
*
Peptide
Download Sample Pages to Get an in-depth Assessment of the Emerging X-linked retinitis pigmentosa Therapies and Key X-linked retinitis pigmentosa Companies: X-linked retinitis pigmentosa Clinical Trials and recent advancements [https://www.delveinsight.com/report-store/x-linked-retinitis-pigmentosa-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
X-linked retinitis pigmentosa Pipeline Therapeutic Assessment
- X-linked retinitis pigmentosa Assessment by Product Type
- X-linked retinitis pigmentosa By Stage
- X-linked retinitis pigmentosa Assessment by Route of Administration
- X-linked retinitis pigmentosa Assessment by Molecule Type
X-linked retinitis pigmentosa Report Section Includes:
*
X-linked retinitis pigmentosa competitive landscape clinical trials
*
X-linked retinitis pigmentosa emerging drugs analysis
*
X-linked retinitis pigmentosa investigational drugs report
*
X-linked retinitis pigmentosa clinical trial trends analysis
*
X-linked retinitis pigmentosa pipeline benchmarking report
*
X-linked retinitis pigmentosa drug pipeline competitive intelligence
*
X-linked retinitis pigmentosa competitive landscape
Major trends shaping the clinical trial and competitive landscape for X-linked retinitis pigmentosa:
*
Focus on Gene Therapies: Rapid growth in AAV-based and other viral vector gene therapies targeting RPGR mutations, aiming to halt or reverse retinal degeneration.
*
Expansion of Clinical Trials: Increasing number of early- and mid-stage trials, often globally conducted to ensure diverse patient recruitment and robust data.
*
Regulatory Support: Orphan Drug, Fast Track, and RMAT designations accelerate development timelines and regulatory review for rare retinal disorders.
*
Emerging Biomarkers and Imaging Techniques: Use of optical coherence tomography (OCT), electroretinography, and genetic biomarkers for patient stratification and precise monitoring of disease progression.
*
Strategic Collaborations: Partnerships between biotech firms, pharmaceutical companies, and academic institutions to pool expertise, technology, and resources.
*
Innovative Delivery Approaches: Focus on subretinal, intravitreal, and optimized vector delivery systems to enhance gene transfer efficiency and safety.
*
Patient-Centric Outcomes: Incorporation of visual function metrics, quality-of-life assessments, and patient-reported outcomes in clinical trial endpoints.
*
Competitive Differentiation: Companies differentiating pipelines based on gene therapy platform, delivery route, stage of development, and mechanism of action.
*
Integration of Real-World Evidence: Leveraging real-world patient data to support regulatory approval, market access, and long-term safety evaluation.
Download X-linked retinitis pigmentosa Sample report to know in detail about the X-linked retinitis pigmentosa treatment market @ X-linked retinitis pigmentosa Therapeutic Assessment [https://www.delveinsight.com/sample-request/x-linked-retinitis-pigmentosa-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
Table of Content
1. Report Introduction
2. Executive Summary
3. X-linked retinitis pigmentosa Current Treatment Patterns
4. X-linked retinitis pigmentosa - DelveInsight's Analytical Perspective
5. Therapeutic Assessment
6. X-linked retinitis pigmentosa Late-Stage Products (Phase-III)
7. X-linked retinitis pigmentosa Mid-Stage Products (Phase-II)
8. Early Stage Products (Phase-I)
9. Pre-clinical Products and Discovery Stage Products
10. Inactive Products
11. Dormant Products
12. X-linked retinitis pigmentosa Discontinued Products
13. X-linked retinitis pigmentosa Product Profiles
14. X-linked retinitis pigmentosa Key Companies
15. X-linked retinitis pigmentosa Key Products
16. Dormant and Discontinued Products
17. X-linked retinitis pigmentosa Unmet Needs
18. X-linked retinitis pigmentosa Future Perspectives
19. X-linked retinitis pigmentosa Analyst Review
20. Appendix
21. Report Methodology
Request the Sample PDF to Get Detailed Insights About the X-linked retinitis pigmentosa Pipeline Reports Offerings [https://www.delveinsight.com/report-store/x-linked-retinitis-pigmentosa-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports Pharma companies by providing comprehensive end-to-end solutions to improve their performance.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Kritika Rehani
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=xlinked-retinitis-pigmentosa-pipeline-analysis-clinical-trials-2025-delveinsight-biogen-4-d-molecular-therapeutics-applied-genetics-technology-corporation]
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: Nevada
Country: United States
Website: https://www.delveinsight.com/
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release X-linked retinitis pigmentosa Pipeline Analysis & Clinical Trials, 2025, DelveInsight | Biogen, 4 D Molecular therapeutics, Applied Genetics Technology Corporation here
News-ID: 4174558 • Views: …
More Releases from ABNewswire

Sydney Business School Alumni Launch Australia's First AI-Driven Wholesale Inves …
Sydney Business School alumni Adnan Tanveer and Adam Newman are launching Investing Platform, Australia's first AI-driven wholesale investment marketplace, in Q1 2026. The platform addresses a key problem: qualified investors currently face seven months and thousands in fees to access a single alternative investment. The founders combine 20+ years of experience with hands-on AI development.
SYDNEY, AUSTRALIA - Investing Platform aims to cut months of friction from wholesale investor access.
Adnan Tanveer…

Sydney Fintech Founder Attending Emergence 2026 Ahead of Platform Launch
Sydney fintech founder Adnan Tanveer is attending Emergence 2026 investment conference (Feb 18-20) as he prepares to launch Investing Platform, Australia's first AI-driven wholesale investment marketplace. Tanveer and co-founder Adam Newman are building infrastructure to cut months of friction from how qualified investors access alternative investments. Platform launches Q1 2026.
SYDNEY, AUSTRALIA - Investing Platform preparing to launch Australia's first AI-driven wholesale investment marketplace.
Adnan Tanveer, co-founder of Investing Platform, is attending…
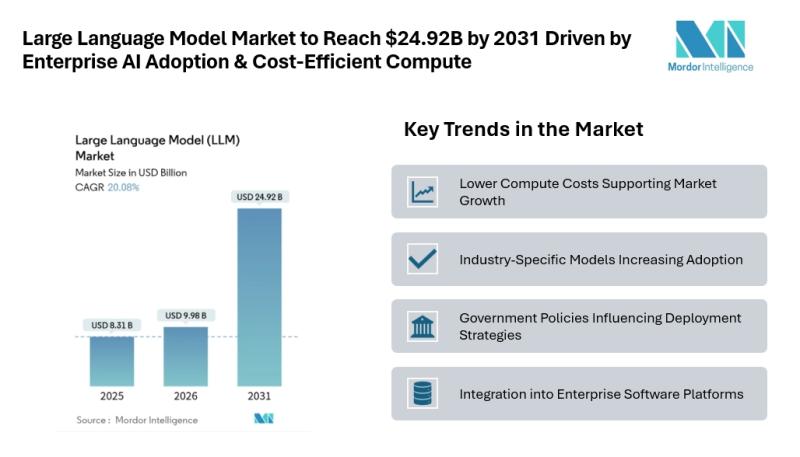
Large Language Model Market to Reach $24.92B by 2031 Driven by Enterprise AI Ado …
Mordor Intelligence has published a new report on the large language model market, offering a comprehensive analysis of trends, growth drivers, and future projections.
Large Language Model Market Outlook
According to Mordor Intelligence, the LLM market size [https://www.mordorintelligence.com/industry-reports/large-language-model-llm-market?utm_source=abnewswire] was valued at USD 8.31 billion in 2025 and is estimated to grow to USD 9.98 billion in 2026, reaching USD 24.92 billion by 2031 at a CAGR of 20.08% during the forecast period.…

Self Employed Tax Software UK: Why Freelancers and Sole Traders Are Switching to …
With Many individuals are seeking software that simplifies tax filing while ensuring full compliance with HMRC requirements. Manual spreadsheets and paper-based calculations are being replaced by real-time, automated systems that give users visibility over their tax position throughout the year. Among the platforms gaining traction is Pie, a UK-based digital tax app built specifically to support self-employed individuals with modern income needs.
LONDON, United Kingdom - February 19, 2026 - Demand…
More Releases for Product
Product Launch
CHENNAI, INDIA - ShiningBot Data Analytics Private Limited, a leader in consumer behavior intelligence, today announced the official launch of ShiningBot version 2.0, a cloud-based platform designed to turn standard Guest WiFi into a sophisticated "intelligence layer" for physical businesses.
In an era where brick-and-mortar establishments struggle to match the data-rich insights of e-commerce, ShiningBot bridges the gap. By leveraging existing WiFi infrastructure, the platform allows Shopping Malls, Hotels, Hospitals, and…
Genstore Ranks #1 Product of the Day on Product Hunt
Los Angeles - September 11, 2025 - Genstore [https://www.genstore.ai/], an AI-native e-commerce platform, ranked #1 Product of the Day on Product Hunt and emerged as one of the week's top-trending products. The recognition underscores strong community support for Genstore's mission to make advanced commerce simple, accessible, and cost-efficient for small and medium-sized businesses worldwide.
Image: https://www.globalnewslines.com/uploads/2025/09/ab03aa9cb9a17e4c42e998d53f216bde.jpg
"Genstore lets anyone start selling online with just a prompt. But of course, that's just the…
Large Volume Parenteral Product Market New Product Development & Latest Trends
The global Large Volume Parenteral (LVP) market is poised for significant growth, projected to reach a value of approximately $12.5 billion in 2024. During the forecast period from 2025 to 2034, the market is expected to expand at a robust Compound Annual Growth Rate (CAGR) of 6.5%, culminating in an estimated market value of $22 billion by 2034.
Exactitude Consultancy., Ltd. released a research report offers a comprehensive examination of the…
Product technology, product usage tips, industry trends
Product Craftsmanship: Yiwu LABON Stationery Co., Ltd. Showcases Superior Craftsmanship in OEM Notebooks
Yiwu LABON Stationery Co., Ltd., established in 2003, has built a reputation for exceptional craftsmanship in the OEM notebook industry. Our factory-based company combines traditional techniques with modern innovation to create notebooks that stand out for their quality and design. Each notebook crafted by Yiwu LABON represents a meticulous process where attention to detail and precision are paramount.…
Product List: The Ultimate Destination for Product and Deal Discovery
Finding the right product or tool to suit your needs can be a daunting task, and securing the best deal on them can be equally challenging.
Each day, plenty of tools are launched, each with unique use cases. Individuals across various industries can benefit from these tools as they simplify their tasks compared to traditional methods. However, it's essential to consider the cost, as some tools are free while others come…
Logistics Packaging Market Enhance Product Safety, Maintain Product Quality, Ext …
MarketResearchReports.Biz presents this most up-to-date research on "Logistics Packaging Market: Global Industry Analysis 2013-2017 and Opportunity Assessment 2018-2028"
The global logistics sector continues to develop at an impressive rate. As a result, the packaging industry is undergoing enormous changes with specified focus on posing innovative packaging tools/products to various industry verticals. Logistics packaging is primarily done to enhance product safety, maintain product quality, extended product storage, and cater to other aspects…
