Press release
Ocular inflammation and pain Pipeline Analysis & Clinical Trials, 2025, DelveInsight | Formosa Pharmaceuticals, Sun Pharma Advanced Research Company Limited, Sun Pharma Global FZE, A.T. Resolve SARL
DelveInsight's, "Ocular Inflammation and Pain - Pipeline Insight, 2025," report provides comprehensive insights about 5+ companies and 5+ pipeline drugs in Ocular Inflammation and Pain pipeline landscape. It covers the pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.DelveInsight's Ocular inflammation and pain Pipeline Clinical Trials Analysis Report provides deep insights to support strategic decision-making for Ocular inflammation and pain pipeline expansion, competitive positioning, and partnership identification in the rare neurodegenerative disease space.
Ocular inflammation and pain Overview:
Inflammation is the body's natural reaction to injury, infection, or irritation. It can also occur in response to harmless substances, such as dust, pollen, or grass, triggering an allergic reaction. Additionally, the immune system may mistakenly attack the body's own tissues, leading to an autoimmune response. Ocular inflammation can arise from infections, allergies, autoimmune conditions, irritation, or trauma affecting the eye or eyelid. Common symptoms include blurred vision, pain, redness, swelling, tearing, and warmth in the eyes, eyelids, or surrounding areas. This condition is frequently observed in patients with systemic inflammatory disorders such as rheumatoid arthritis, lupus, sarcoidosis, Wegener's granulomatosis, Sjogren's syndrome, and others.
Download our report @ https://www.delveinsight.com/report-store/ocular-inflammation-and-pain-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr
"Ocular inflammation and pain Pipeline Insight 2025" report by DelveInsight provides a comprehensive analysis of the ongoing clinical development activities and growth prospects across the Ocular inflammation and pain Therapeutics Market.
Key Takeaways from the Ocular inflammation and pain Pipeline Report
*
DelveInsight's Ocular Inflammation and Pain Pipeline Report highlights a dynamic landscape, with over 5 companies developing more than 5 therapeutic candidates for the treatment of ocular inflammation and pain.
*
A corticosteroid ophthalmic suspension, approved on March 4, 2024, is indicated for post-operative inflammation and pain following eye surgery. Clinical studies reported ocular adverse reactions in greater than or equal to 1% of patients, including eye inflammation (2%), corneal edema (2%), anterior chamber inflammation (2%), cystoid macular edema (2%), elevated intraocular pressure (1%), photophobia (1%), and vitreous detachment (1%).
*
MIEBO, approved on May 18, 2023, is a first-in-class therapy for the signs and symptoms of dry eye disease (DED), a multifactorial, age-related, chronic progressive disorder of the ocular surface. MIEBO is specifically indicated for alleviating both the signs and symptoms of DED.
*
Leading companies in this space include Formosa Pharmaceuticals, Inc., Sun Pharma Advanced Research Company Limited, Sun Pharma Global FZE, A.T. Resolve SARL, Tarsius Pharma, SALVAT, and others, all working to expand treatment options. Promising pipeline candidates include APP13007 and additional therapies aimed at improving the ocular inflammation and pain treatment landscape.
Ocular inflammation and pain Pipeline Analysis
The Ocular inflammation and pain pipeline insights report 2025, provides insights into:
*
Provides comprehensive insights into key companies developing therapies in the Ocular inflammation and pain Market.
*
Categorizes Ocular inflammation and pain therapeutic companies by development stage: early, mid, and late-stage.
*
Highlights major companies involved in targeted therapy development, including both active and inactive (paused/discontinued) projects.
*
Reviews emerging Ocular inflammation and pain drugs under development based on:
*
Stage of development
*
Ocular inflammation and pain Route of administration
*
Target receptor
*
Monotherapy vs. combination therapy
*
Ocular inflammation and pain Mechanism of action
*
Molecular type
*
Offers detailed analysis of:
*
Company-to-company and company-academia collaborations
*
Ocular inflammation and pain Licensing agreements
*
Funding and investment activities supporting future Ocular inflammation and pain market advancement.
Request for a sample report @ https://www.delveinsight.com/sample-request/ocular-inflammation-and-pain-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr
Ocular inflammation and pain Emerging Drugs
*
APP13007: Formosa pharmaceuticals
APP13007, developed using our proprietary APNT formulation platform, is an aqueous ophthalmic nanosuspension designed to manage post-operative pain and inflammatory eye conditions. Its active ingredient is a potent corticosteroid with over 30 years of proven efficacy and safety in dermatological applications across the U.S., Europe, and Japan. APP13007 represents the first ophthalmic application of this well-established compound. Following the completion of Phase II studies in 2020, it advanced to Phase III clinical trials in the U.S. in March 2022.
Ocular inflammation and pain Companies
More than five key companies are developing therapies for ocular inflammation and pain, with Formosa Pharmaceuticals leading the field with candidates currently in Phase III trials, the most advanced stage of development.
DelveInsight's report covers around 5+ products under different phases of Ocular inflammation and pain clinical trials like
*
Ocular inflammation and pain Late stage Therapies (Phase III)
*
Ocular inflammation and pain Mid-stage Therapies (Phase II)
*
Ocular inflammation and pain Early-stage Therapies (Phase I)
*
Ocular inflammation and pain Pre-clinical and Ocular inflammation and pain Discovery stage Therapies
*
Ocular inflammation and pain Discontinued & Inactive Therapies
Ocular inflammation and pain pipeline report provides the Ocular inflammation and pain therapeutic assessment of the pipeline drugs by the Route of Administration. Products have been categorized under various ROAs such as
*
Intravenous
*
Subcutaneous
*
Oral
*
Intramuscular
Ocular inflammation and pain Products have been categorized under various Molecule types such as
*
Monoclonal antibody
*
Small molecule
*
Peptide
Download Sample Pages to Get an in-depth Assessment of the Emerging Ocular inflammation and pain Therapies and Key Ocular inflammation and pain Companies: Ocular inflammation and pain Clinical Trials and recent advancements [https://www.delveinsight.com/report-store/ocular-inflammation-and-pain-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
Ocular inflammation and pain Pipeline Therapeutic Assessment
- Ocular inflammation and pain Assessment by Product Type
- Ocular inflammation and pain By Stage
- Ocular inflammation and pain Assessment by Route of Administration
- Ocular inflammation and pain Assessment by Molecule Type
Ocular inflammation and pain Report Section Includes:
*
Ocular inflammation and pain competitive landscape clinical trials
*
Ocular inflammation and pain emerging drugs analysis
*
Ocular inflammation and pain investigational drugs report
*
Ocular inflammation and pain clinical trial trends analysis
*
Ocular inflammation and pain pipeline benchmarking report
*
Ocular inflammation and pain drug pipeline competitive intelligence
*
Ocular inflammation and pain competitive landscape
Major trends shaping the clinical trial and competitive landscape for Ocular inflammation and pain:
*
Shift Toward Novel and Targeted Therapies: Increased development of corticosteroids, biologics, antisense oligonucleotides, and regenerative therapies to treat underlying inflammation rather than just symptoms.
*
Expansion of Clinical Trials: Growth in early- and mid-stage trials, exploring multiple mechanisms, formulations, and combination therapies.
*
Regulatory Support: Orphan Drug, Fast Track, and RMAT designations facilitating expedited development for rare or severe ocular conditions.
*
Strategic Collaborations: Partnerships among biotech, pharmaceutical companies, and academic institutions to accelerate R&D and share technological expertise.
*
Innovation in Drug Delivery and Formulation: Focus on nanosuspensions, topical applications, sustained-release formulations, and ocular implants to improve efficacy and patient compliance.
*
Emerging Biomarkers and Patient-Centric Endpoints: Incorporation of clinical and imaging biomarkers, patient-reported outcomes, and vision-related quality-of-life measures to enhance trial precision and relevance.
*
Competitive Differentiation: Companies distinguishing pipelines by mechanism of action, route of administration, molecular type, and stage of development.
*
Integration of Real-World Evidence: Use of real-world data to support regulatory approval, reimbursement, and post-market monitoring.
Download Ocular inflammation and pain Sample report to know in detail about the Ocular inflammation and pain treatment market @ Ocular inflammation and pain Therapeutic Assessment [https://www.delveinsight.com/sample-request/ocular-inflammation-and-pain-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
Table of Content
1. Report Introduction
2. Executive Summary
3. Ocular inflammation and pain Current Treatment Patterns
4. Ocular inflammation and pain - DelveInsight's Analytical Perspective
5. Therapeutic Assessment
6. Ocular inflammation and pain Late-Stage Products (Phase-III)
7. Ocular inflammation and pain Mid-Stage Products (Phase-II)
8. Early Stage Products (Phase-I)
9. Pre-clinical Products and Discovery Stage Products
10. Inactive Products
11. Dormant Products
12. Ocular inflammation and pain Discontinued Products
13. Ocular inflammation and pain Product Profiles
14. Ocular inflammation and pain Key Companies
15. Ocular inflammation and pain Key Products
16. Dormant and Discontinued Products
17. Ocular inflammation and pain Unmet Needs
18. Ocular inflammation and pain Future Perspectives
19. Ocular inflammation and pain Analyst Review
20. Appendix
21. Report Methodology
Request the Sample PDF to Get Detailed Insights About the Ocular inflammation and pain Pipeline Reports Offerings [https://www.delveinsight.com/report-store/ocular-inflammation-and-pain-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports Pharma companies by providing comprehensive end-to-end solutions to improve their performance.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Kritika Rehani
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=ocular-inflammation-and-pain-pipeline-analysis-clinical-trials-2025-delveinsight-formosa-pharmaceuticals-sun-pharma-advanced-research-company-limited-sun-pharma-global-fze-at-resolve-sarl]
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: Nevada
Country: United States
Website: https://www.delveinsight.com/
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Ocular inflammation and pain Pipeline Analysis & Clinical Trials, 2025, DelveInsight | Formosa Pharmaceuticals, Sun Pharma Advanced Research Company Limited, Sun Pharma Global FZE, A.T. Resolve SARL here
News-ID: 4174557 • Views: …
More Releases from ABNewswire
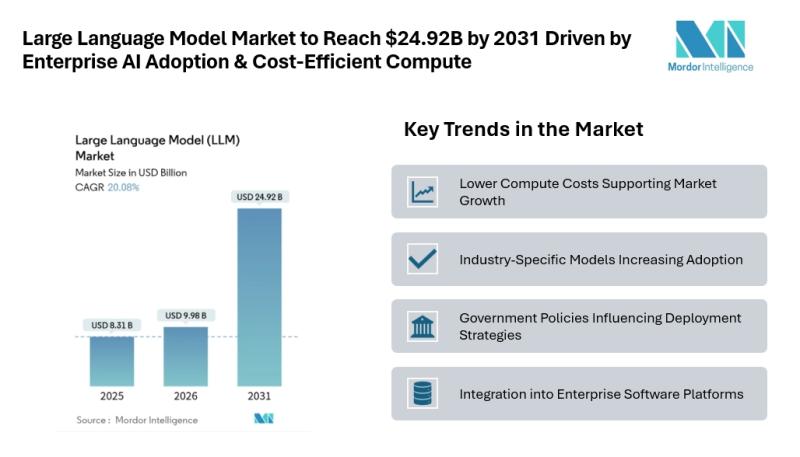
Large Language Model Market to Reach $24.92B by 2031 Driven by Enterprise AI Ado …
Mordor Intelligence has published a new report on the large language model market, offering a comprehensive analysis of trends, growth drivers, and future projections.
Large Language Model Market Outlook
According to Mordor Intelligence, the LLM market size [https://www.mordorintelligence.com/industry-reports/large-language-model-llm-market?utm_source=abnewswire] was valued at USD 8.31 billion in 2025 and is estimated to grow to USD 9.98 billion in 2026, reaching USD 24.92 billion by 2031 at a CAGR of 20.08% during the forecast period.…

Self Employed Tax Software UK: Why Freelancers and Sole Traders Are Switching to …
With Many individuals are seeking software that simplifies tax filing while ensuring full compliance with HMRC requirements. Manual spreadsheets and paper-based calculations are being replaced by real-time, automated systems that give users visibility over their tax position throughout the year. Among the platforms gaining traction is Pie, a UK-based digital tax app built specifically to support self-employed individuals with modern income needs.
LONDON, United Kingdom - February 19, 2026 - Demand…
CivicMail.org Reinvents Postcard Campaigns for Grassroots Advocacy
CivicMail.org aims to bring civic engagement back to basics through the power of pen, paper, and postage.
Image: https://www.abnewswire.com/upload/2026/02/2addd1e9e0381d7e2262e1edbb064123.jpg
CivicMail.org [https://civicmail.org/] has announced its launch to help Americans send real, physical postcards to their elected officials with just a few clicks, delivering personalized messages directly to the desks of decision-makers at the local, state, and federal levels.
Research shows [https://www.concordia.ca/news/stories/2021/09/24/personalized-messages-are-more-likely-to-get-a-response-from-politicians-new-research-finds.html] that physical mail carries more weight with elected officials than petitions, emails, or…

New Children's Story: The Story of Sharin' Bear
A Heartfelt Message Of Courage, Kindness, And The True Meaning Of Giving
A pleasant new story for children, The Story of Sharin' Bear by Sharon Woods , introduces families to a lovable little cub whose journey of bravery and compassion changes him into a representation of sharing for children globally.
Entrenched in adventure, innocence, and emotional growth, this uplifting tale offers an unforgettable reminder that even the smallest acts of kindness can…
More Releases for Ocular
Ocular Prosthesis Market Forecast Through 2026-2033: Emerging Trends, Market Dri …
The qualitative latest Research report (2026-2033) on the Ocular Prosthesis Market by Coherent Market Insights Provides a deep dive into key market trends, drivers, challenges, and the competitive landscape. It analyzes market size, revenue, production, and CAGR using validated methodologies to ensure precision. The report highlights tech innovation, pricing trends, consumer behavior, and investment potential - empowering businesses to make informed, strategic moves.
➤ Request a Sample Copy (Complete TOC, Tables…
How the Ocular Pain Market Is Growing in 2025 | What's New Treatments Are Coming …
According to the IMARC Group, the ocular pain market is expected to exhibit a CAGR of 7.66% during 2025-2035. This can be attributed to the growing use of services in teleophthalmology, which facilitates remote consultations and enhances patient adherence.
Ocular pain is the discomfort or distress experienced within or around the eye. The ocular pain market is experiencing significant growth driven by multiple factors. Primarily, the increasing prevalence of conditions such…
Neuropathic Ocular Pain Market Is Booming Worldwide 2025-2032 | OKYO Pharma, Lim …
Latest Report, titled "Neuropathic Ocular Pain Market" Trends, Share, Size, Growth, Opportunity and Forecast 2025-2032, by Coherent Market Insights offers a comprehensive analysis of the industry, which comprises insights on the market analysis. The report also includes competitor and regional analysis, and contemporary advancements in the market.
The report features a comprehensive table of contents, figures, tables, and charts, as well as insightful analysis. The Neuropathic Ocular Pain market has been…
Neuropathic Ocular Pain Market Detailed In New Research Report 2024 |OKYO Pharma …
A recent report from Coherent Market Insight titled "Neuropathic Ocular Pain Market: Industry Trends, Share, Size, Growth, Opportunity, and Forecast 2024-2031" presents a thorough examination of the industry landscape, offering insights into market analysis and trends. Alongside competitor and regional analysis, the report delves into contemporary advancements within the market.
Global Neuropathic Ocular Pain market is estimated to be valued at US$ 197.1 million in 2023 and is expected to…
Ocular Neuropathic Pain Market to witness Robust Expansion by 2031 - OKYO Pharma …
Ocular neuropathic pain (ONP) is chronic eye pain caused by nerve damage or dysfunction. It can result from various conditions, such as corneal nerve injury, ocular surgeries, infections, or systemic diseases like diabetes. Patients experience persistent, burning, or stabbing eye pain, often without visible signs of inflammation. Diagnosis involves detailed patient history, clinical examination, and sometimes specialized tests. Treatment focuses on managing pain and may include topical or systemic medications,…
Ocular Implant Market - Redefining Ophthalmic Solutions: Choose Ocular Implants …
Newark, New Castle, USA: The "Ocular Implant Market" provides a value chain analysis of revenue for the anticipated period from 2022 to 2030. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors
Ocular Implant Market: https://www.growthplusreports.com/report/ocular-implant-market/7864
This latest report researches the industry structure, sales, revenue,…
