Press release
Global Biosimilar Market Size, Share, Growth And Trends Report 2025-2033
Overview of the Biosimilar Market:The biosimilar market is a rapidly growing segment of the pharmaceutical industry that focuses on the development and commercialization of biosimilars-biological products that are highly similar to already approved reference biologics. These products are designed to offer similar safety and efficacy profiles as their reference counterparts but are typically more affordable, providing a cost-effective alternative for patients and healthcare systems. The increasing prevalence of chronic diseases, coupled with the rising costs of biologic therapies, has fueled the demand for biosimilars. Regulatory frameworks in various regions, including the U.S. and Europe, have evolved to facilitate the approval of biosimilars, ensuring rigorous evaluation while promoting market access. As more biosimilars gain approval and enter the market, they are expected to enhance competition, reduce healthcare expenditures, and improve patient access to essential biologic treatments. The biosimilar market is poised for significant growth, driven by advancements in biotechnology, increasing investment in research and development, and the growing acceptance of biosimilars among healthcare professionals and patients.
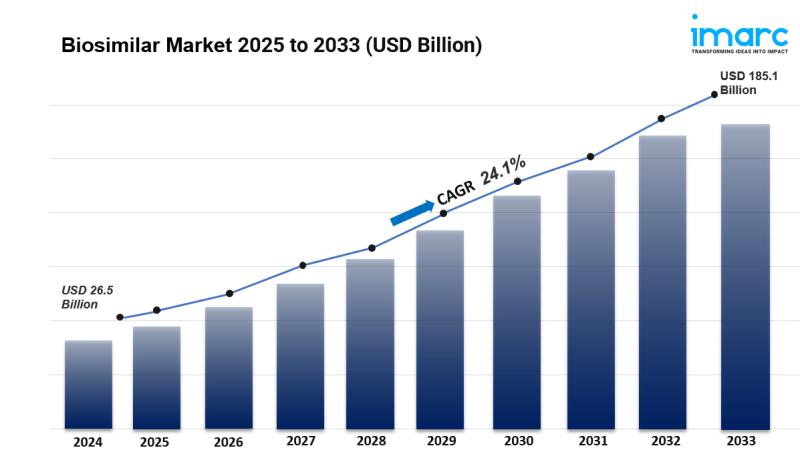
The global biosimilar market size was valued at USD 26.5 Billion in 2024 and is expected to reach USD 185.1 Billion by 2033, exhibiting a CAGR of 24.1% from 2025-2033. Europe currently dominates the market. The expiration of patents for major biological drugs, growing awareness about the efficacy and cost-effectiveness of biosimilars, the rising prevalence of chronic diseases worldwide, and continual advancements in biopharmaceutical manufacturing technologies are some of the major factors boosting the biosimilar market share.
Buy Now: https://www.imarcgroup.com/checkout?id=497&method=1670
Key Highlights of the Biosimilar Market:
Regulatory Advancements: Regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have established clear guidelines for the approval of biosimilars, which has significantly boosted market confidence. These regulations ensure that biosimilars undergo rigorous testing to demonstrate their similarity to reference products in terms of safety, efficacy, and quality. The streamlined approval processes have led to an increase in the number of biosimilars entering the market, fostering competition and driving down prices for patients.
Cost-Effectiveness and Accessibility: One of the primary drivers of the biosimilar market is the potential for cost savings. Biosimilars are generally priced lower than their reference biologics, making them more accessible to patients and healthcare systems. This affordability is particularly important in the context of rising healthcare costs and increasing demand for biologic therapies. By providing a more cost-effective alternative, biosimilars can help alleviate the financial burden on patients, insurers, and healthcare providers. As awareness of the benefits of biosimilars grows,
Growing Pipeline and Therapeutic Areas: The biosimilar market is witnessing a robust pipeline of products across various therapeutic areas, including oncology, autoimmune diseases, and diabetes. Major pharmaceutical companies and biotechnology firms are investing heavily in the development of biosimilars, recognizing the significant market potential.
Market Competition and Innovation: The entry of biosimilars into the market is intensifying competition among pharmaceutical companies, leading to innovation in both product development and marketing strategies. As biosimilars become more prevalent, original biologic manufacturers are also responding by reducing prices, improving formulations, and enhancing patient support programs.
Patient and Physician Acceptance: The acceptance of biosimilars among patients and healthcare professionals is crucial for the market's growth. Education and awareness campaigns are essential to inform stakeholders about the benefits, safety, and efficacy of biosimilars. As healthcare providers become more familiar with biosimilars and their clinical applications, they are more likely to prescribe these products.
Regional Market Dynamics: The biosimilar market is characterized by varying dynamics across different regions. North America and Europe are currently the leading markets for biosimilars, driven by established regulatory frameworks and high healthcare expenditures. However, emerging markets in Asia-Pacific and Latin America present significant growth opportunities due to increasing healthcare access, rising chronic disease prevalence, and the need for affordable treatment options.
Impact of Patent Expirations: The expiration of patents for several blockbuster biologics is creating a favorable environment for the biosimilar market. As patents expire, the original manufacturers face competition from biosimilars, leading to price reductions and increased accessibility for patients.
Future Outlook and Challenges: The future of the biosimilar market appears promising, with continued growth expected in the coming years. However, several challenges remain, including the need for ongoing education and awareness to overcome misconceptions about biosimilars. Additionally, navigating the complexities of regulatory requirements and intellectual property issues can pose hurdles for biosimilar developers.
Factors Affecting the Growth of the Biosimilar Market Industry:
Increasing Acceptance of Biosimilars:
The biosimilar market is growing quickly. This growth comes from more healthcare providers, payers, and patients accepting these products. As the healthcare landscape changes, people are learning about biosimilars and their cost-effective options compared to expensive biologics. Clinical evidence shows that biosimilars are safe and effective. This evidence helps ease concerns about using them. Also, regulatory agencies like the FDA and EMA have set clear approval pathways for biosimilars. This boosts confidence in their quality and effectiveness. As drug costs rise globally, biosimilars offer a solution to improve access to vital therapies.
Expanding Therapeutic Applications:
The biosimilar market is growing its range of uses. Initially, biosimilars focused on oncology and inflammatory diseases. Now, they are expanding into metabolic disorders, ophthalmology, and rare diseases. This shift is due to more biologic patents expiring. This gives biosimilar makers chances to enter new markets. Also, new biomanufacturing technologies are helping create high-quality biosimilars. These can compete well with original biologics in many areas.
Strategic Collaborations and Partnerships:
Strategic partnerships among pharmaceutical companies, biotech firms, and research institutions are growing in the biosimilar market. These alliances help share expertise, resources, and technologies. By working together, companies can reduce the costs of research and development. They can also navigate regulations better and expand their market reach. For example, large pharmaceutical firms often team up with smaller biotech companies that focus on biosimilars. This allows them to access innovative technologies and speed up product launches.
Request to Get the Sample Report: https://www.imarcgroup.com/biosimilar-market/requestsample
Biosimilar Market Report Segmentation:
Breakup By Molecule:
• Infliximab
• Insulin Glargine
• Epoetin Alfa
• Etanercept
• Filgrastim
• Somatropin
• Rituximab
• Follitropin Alfa
• Adalimumab
• Pegfilgrastim
• Trastuzumab
• Bevacizumab
• Others
Infliximab accounts for the majority of shares due to its widespread use in treating chronic autoimmune diseases such as rheumatoid arthritis and Crohn's disease.
Breakup By Indication:
• Auto-Immune Diseases
• Blood Disorder
• Diabetes
• Oncology
• Growth Deficiency
• Female Infertility
• Others
Autoimmune diseases dominate the market as biologics, and biosimilars are highly effective in managing conditions like rheumatoid arthritis, psoriasis, and inflammatory bowel disease.
Breakup By Manufacturing Type:
• In-house Manufacturing
• Contract Manufacturing
In-house manufacturing represents the majority of shares because it enables better control over production quality and reduces reliance on third-party manufacturers, ensuring compliance with strict regulatory standards.
Breakup By Region:
• Europe
o Germany
o France
o Italy
o Spain
o United Kingdom
o Rest of Europe
• United States
• Japan
• India
• South Korea
• Rest of the World
Europe holds the leading position due to its well-established regulatory pathways for biosimilars and strong government support for biosimilar adoption.
Top Biosimilar Market Leaders:
The biosimilar market research report outlines a detailed analysis of the competitive landscape, offering in-depth profiles of major companies.
Some of the key players in the market are:
• Sandoz International GmbH
• Pfizer Inc.
• Teva Pharmaceutical Industries Limited
• Celltrion Inc.
• Biocon Limited
• Samsung Biologics
• Amgen, Inc.
• Dr. Reddy's Laboratories Limited
• Stada Arzneimittel Ag.
Speak to An Analyst: https://www.imarcgroup.com/request?type=report&id=497&flag=C
If you need specific information that is not currently within the scope of the report, we will provide it to you as a part of the customization.
About Us
IMARC Group is a global management consulting firm that helps the world's most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services.
IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact US:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
United States: +1-201971-6302
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Global Biosimilar Market Size, Share, Growth And Trends Report 2025-2033 here
News-ID: 4173495 • Views: …
More Releases from IMARC Group
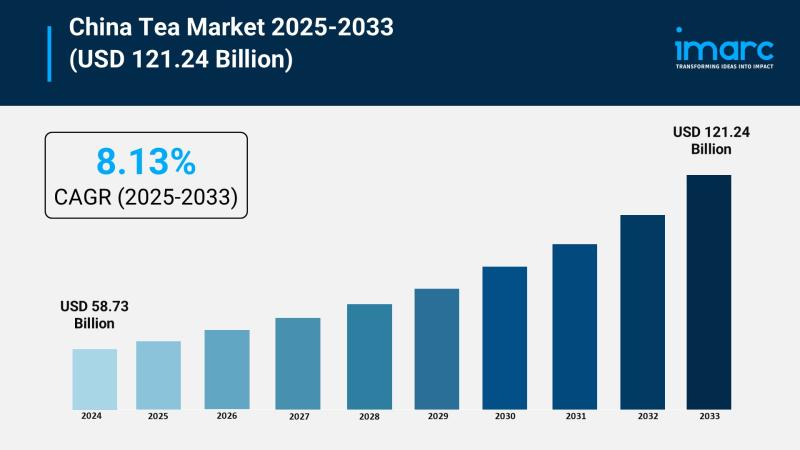
China Tea Market Forecast CAGR of 8.13%, Innovation Trends, and Strategic Insigh …
Market Overview
The China tea market was valued at USD 58.73 Billion in 2024 and is projected to reach USD 121.24 Billion by 2033, growing at a CAGR of 8.13% during 2025-2033. Growth is driven by rising health consciousness, premium product trends, government support, and expanding online retail. Innovation in flavors and packaging attracts younger consumers and global buyers, expanding the market.
Study Assumption Years
• Base Year: 2024
• Historical Year/Period: 2019-2024
• Forecast Year/Period: 2025-2033
China Tea…
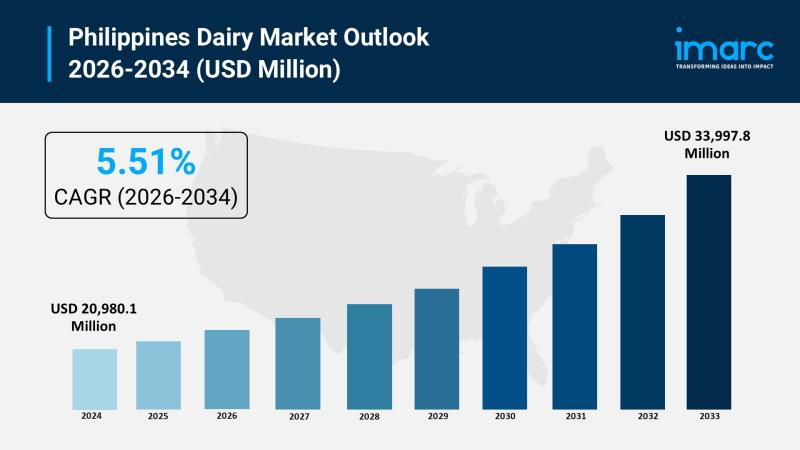
Philippines Dairy Market 2026: Expected to Reach USD 33,997.8 Million by 2034
Market Overview
The Philippines dairy market reached a size of USD 20,980.1 Million and is anticipated to grow to USD 33,997.8 Million by 2034 with a significant growth rate of 5.51%. This expansion is driven by rising demand for nutritious and diverse dairy products, rapid urbanization, increased disposable incomes, improved retail infrastructure, and strong government initiatives promoting local dairy production. Health-conscious consumers and expanding food service sectors further fuel this growth…
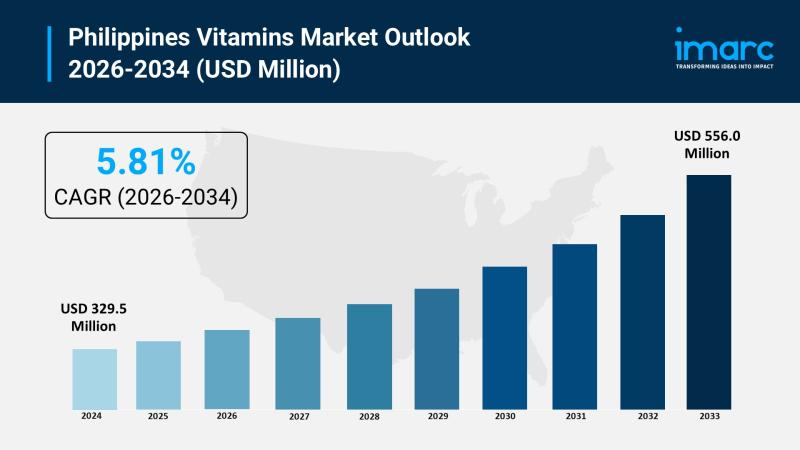
Philippines Vitamins Market 2026 | Projected to Reach USD 556.0 Million by 2034
Market Overview
The Philippines vitamins market was valued at USD 329.5 Million in 2025 and is projected to reach USD 556.0 Million by 2034, growing steadily over the forecast period. The market's growth is driven by increasing health consciousness, a rising geriatric population, and escalating demand for supplements that support immunity, energy, and overall wellness due to proactive health measures. The forecast period for this expansion is 2026-2034, with a CAGR…
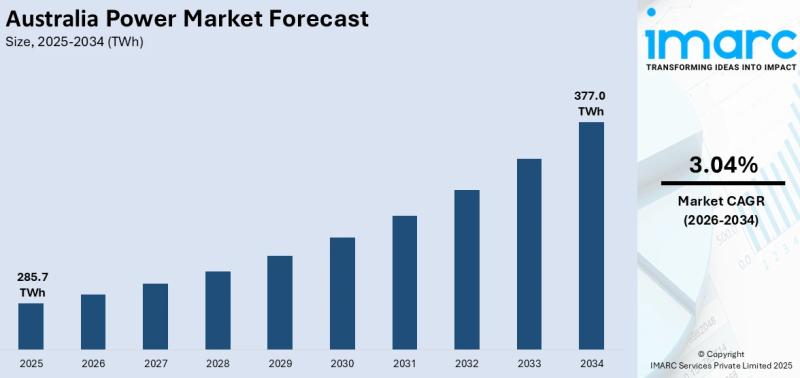
Australia Power Market Projected to Reach TWH 377 by 2034
Market Overview
The Australia power market size reached 285.7 TWh in 2025 and is projected to grow to 377.0 TWh by 2034, with a CAGR of 3.04% during the forecast period of 2026-2034. This growth is driven by rising renewable energy adoption, increased electricity demand, grid modernization, battery storage expansion, transition from coal plants, and government incentives for clean power. Key strategies such as virtual power plant integration and investments in…
More Releases for Biosimilar
Interchangeable Biosimilar Humira Market Share Driven by Biologic Therapy Adopti …
Interchangeable Biosimilar Humira Market
The global market for Interchangeable Biosimilar Humira was valued at US$ million in the year 2024 and is projected to reach a revised size of US$ million by 2031, growing at a CAGR of %during the forecast period
View sample report
https://reports.valuates.com/request/sample/QYRE-Auto-33I15005/Global_Interchangeable_Biosimilar_Humira_Market_Research_Report_2023
The Interchangeable Biosimilar Humira Market is experiencing significant market growth as healthcare providers and patients increasingly adopt biosimilar therapies for autoimmune and inflammatory conditions. Market trends indicate rising…
Key Trend Reshaping the Biosimilar Monoclonal Antibodies Market in 2025: Advance …
What Are the Projections for the Size and Growth Rate of the Biosimilar Monoclonal Antibodies Market?
In recent times, the biosimilar monoclonal antibodies sector has experienced a swift expansion. The market size, which stands at $8.04 billion in 2024, is projected to climb to $9.25 billion in 2025, marking a compound annual growth rate (CAGR) of 15.1%. Factors such as expired patents, an increased understanding of biosimilars, governmental strategies, heightened financial…
Key Trend Reshaping the Biosimilar Monoclonal Antibodies Market in 2025: Advance …
What Are the Projections for the Size and Growth Rate of the Biosimilar Monoclonal Antibodies Market?
In recent times, the biosimilar monoclonal antibodies sector has experienced a swift expansion. The market size, which stands at $8.04 billion in 2024, is projected to climb to $9.25 billion in 2025, marking a compound annual growth rate (CAGR) of 15.1%. Factors such as expired patents, an increased understanding of biosimilars, governmental strategies, heightened financial…
Biosimilar Market Treating More for Less: The Booming Infliximab Biosimilar Mark …
Infliximab Biosimilar Market worth $ XX Million by 2030 - Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Infliximab Biosimilar Market- by Application (Crohn's Disease, Psoriatic Arthritis, Rheumatoid Arthritis, Ulcerative Colitis, Ankylosing Spondylitis, Plaque Psoriasis and Others), End User (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy and Other Direct Distribution Channels), Trends, Industry Competition Analysis, Revenue and Forecast To 2030."
Get…
Biosimilar Monoclonal Antibodies Market
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the " "Global Biosimilar Monoclonal Antibodies Market by Product (infliximab, trastuzumab, rituximab, adalimumab, bevacizumab, cetuximab, ranibizumab, denosumab, eculizumab, and other pipeline products), Indication (oncology, inflammatory & autoimmune disorders, chronic diseases, blood disorders, and other indications), Clinical Trial/Pipeline Analysis, Future Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
The Biosimilar Monoclonal Antibodies Market Size is valued at 5.02…
Infliximab Biosimilar Insight, 2022 | DelveInsight
DelveInsight's, "Infliximab Biosimilar Insight, 2022" report provides comprehensive insights about 35+ companies and 45+ marketed and pipeline drugs in Infliximab Biosimilars landscape. It covers the marketed and pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Interested to know more about the functioning of…