Press release
Post-Marketing Pharmacovigilance And Medical Information Market Landscape 2025: Forecast Data and Growth Strategy Insights
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.What Is the Expected CAGR for the Post-Marketing Pharmacovigilance And Medical Information Market Through 2025?
The size of the market for post-marketing pharmacovigilance and medical information has seen swift expansion in recent years. This market is expected to surge from a worth of $6.16 billion in 2024 to $6.90 billion in 2025, experiencing a compound annual growth rate (CAGR) of 11.9%. Factors contributing to the growth during the historic period include the increased incorporation of AI in pharmacovigilance activities, an escalating demand for contracted drug safety services, the increased intricacy of biopharmaceutical products, a heightened focus on patient-reported outcomes, and fostering collaboration between regulatory bodies and the industry.
What's the Projected Size of the Global Post-Marketing Pharmacovigilance And Medical Information Market by 2029?
In the coming years, the market size for post-marketing pharmacovigilance and medical information is projected to see a swift expansion, reaching a value of $10.68 billion in 2029 at a compound annual growth rate (CAGR) of 11.6%. This growth during the forecast period can be attributed to factors such as progress in drug safety analytic tools, growing usage of cloud-based pharmacovigilance solutions, an increasing number of novel therapeutic launches, an escalating focus on proactive risk management strategies, and burgeoning pharmacovigilance needs in emerging markets. The forecast period would also witness key trends including the advancement in automatic detection of adverse events, development of integrated pharmacovigilance platforms, innovation in real-time safety data monitoring, progress in patient engagement tools for safety reporting, and the creation of predictive analytics for the assessment of drug risks.
View the full report here:
https://www.thebusinessresearchcompany.com/report/post-marketing-pharmacovigilance-and-medical-information-global-market-report
Top Growth Drivers in the Post-Marketing Pharmacovigilance And Medical Information Industry: What's Accelerating the Market?
The escalating number of negative drug reactions is projected to fuel the expansion of the post-marketing pharmacovigilance and medical information market. Negative drug reactions pertain to damaging or unintended results that transpire when a drug is utilized at its recommended dosage for preventative, diagnostic, or therapeutic purposes. The frequency of these reactions increases as the elderly demographic increasingly requires medication for multiple chronic diseases, leading to the concurrent use of numerous drugs, which raises the possibility of detrimental drug interactions and unforeseen side effects. Post-marketing pharmacovigilance and medical information aid in managing negative drug reactions by identifying, monitoring, and disseminating information on possible side effects post-drug launch, ensuring its safety and efficacy. For example, Navikenz, a US-based IT services firm specializing in artificial intelligence, estimated in January 2023, that in the US, negative drug reactions are projected to result in 200,000 to 400,000 deaths annually, surpassing the combined deaths from stroke and diabetes, with an approximate 2.7 million cases each year resulting in over 100,000 hospital stays and more than 15,000 deaths. Thus, the escalating number of adverse drug reactions stimulates the growth of the post-marketing pharmacovigilance and medical information market.
Get your free sample here:
https://www.thebusinessresearchcompany.com/sample.aspx?id=27393&type=smp
What Are the Key Trends Driving Post-Marketing Pharmacovigilance And Medical Information Market Growth?
In the post-marketing pharmacovigilance and medical information market, principal companies are concentrating on developing more sophisticated solutions that utilize artificial intelligence. These AI-integrated solutions, such as pharmacovigilance workflows, aim to improve the precision of adverse event detection and provide real-time safety surveillance for improved patient health outcomes. In January 2024, for instance, UK cloud-based Software-as-a-Service (SaaS) company PubHive Ltd., launched a centralized Summary of Product Characteristics (SmPC) management platform. Its purpose is to serve life science companies and research organizations, amalgamating important drug safety data in a singular, easy-to-access database. The system automates procedure for monitoring literature and regulatory reporting, facilitates team cooperation, and simplifies compliance processes using advanced AI skills. These improvements allow pharmacovigilance teams to work more effectively and proactively, ensuring patient safety and regulatory compliance.
What Are the Main Segments in the Post-Marketing Pharmacovigilance And Medical Information Market?
The post-marketing pharmacovigilance and medical information market covered in this report is segmented
1) By Type: Spontaneous Reporting, Intensified Adverse Drug Reaction (ADR) Reporting, Targeted Spontaneous Reporting, Cohort Event Monitoring, Electronic Health Record (EHR) Mining
2) By Product: Books, Online Media, Journals
3) By End User: Hospitals, Research Organizations, Other End-Users
Subsegments:
1) By Spontaneous Reporting: Consumer Reporting, Healthcare Professional Reporting, Regulatory Authority Reporting, Pharmaceutical Company Reporting, Digital App-Based Reporting
2) By Intensified Adverse Drug Reaction (ADR) Reporting: Hospital-Based Surveillance, Program-Specific Monitoring, Disease-Specific Monitoring, Product-Specific Monitoring, Real-Time Monitoring Systems
3) By Targeted Spontaneous Reporting: Risk Population-Based Reporting, Therapeutic Class-Based Reporting, Adverse Drug Reaction Focused Reporting, Region-Specific Reporting, Healthcare Setting-Specific Reporting
4) By Cohort Event Monitoring: Prospective Cohort Monitoring, Retrospective Cohort Monitoring, Active Follow-Up Monitoring, New Drug User Monitoring, Disease Registry-Linked Monitoring
5) By Electronic Health Record (EHR) Mining: Natural Language Processing-Based Mining, Artificial Intelligence (AI) And Machine Learning Algorithms, Rule-Based Signal Detection, Longitudinal Patient Data Analysis, Integrated Hospital Data Systems
Tailor your insights and customize the full report here:
https://www.thebusinessresearchcompany.com/customise?id=27393&type=smp
Which Top Companies are Driving Growth in the Post-Marketing Pharmacovigilance And Medical Information Market?
Major companies operating in the post-marketing pharmacovigilance and medical information market are Cencora Inc., Cardinal Health Inc., accenture* plc, Sanofi S.A., Thermo Fisher Scientific Inc., Capgemini SE, Merck & Co. Inc., Cognizant Technology Solutions Corporation, IQVIA Holdings Inc., HCL Technologies Limited, Laboratory Corporation of America Holdings, ICON plc, WuXi AppTec Co. Ltd., Syneos Health Inc., Genpact Limited, Charles River Laboratories International Inc., Parexel International Corporation, Avalere Health LLC, Quanticate Limited, Inizio Consulting LLC.
Which Regions Will Dominate the Post-Marketing Pharmacovigilance And Medical Information Market Through 2029?
North America was the largest region in the post-marketing pharmacovigilance and medical information market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the post-marketing pharmacovigilance and medical information market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa.
Purchase the full report today:
https://www.thebusinessresearchcompany.com/purchaseoptions.aspx?id=27393
This Report Supports:
1.Business Leaders & Investors - To identify growth opportunities, assess risks, and guide strategic decisions.
2.Manufacturers & Suppliers - To understand market trends, customer demand, and competitive positioning.
3.Policy Makers & Regulators - To track industry developments and align regulatory frameworks.
4.Consultants & Analysts - To support market entry, expansion strategies, and client advisory work.
Speak With Our Expert:
Saumya Sahay,
Americas: +1 310-496-7795,
Asia: +44 7882 955267 & +91 8897263534,
Europe: +44 7882 955267,
Email: saumyas@tbrc.info
The Business Research Company - www.thebusinessresearchcompany.com
Follow Us On:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
Learn More About The Business Research Company
With over 15,000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Our flagship product, the Global Market Model delivers comprehensive and updated forecasts to support informed decision-making.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Post-Marketing Pharmacovigilance And Medical Information Market Landscape 2025: Forecast Data and Growth Strategy Insights here
News-ID: 4171721 • Views: …
More Releases from The Business Research Company
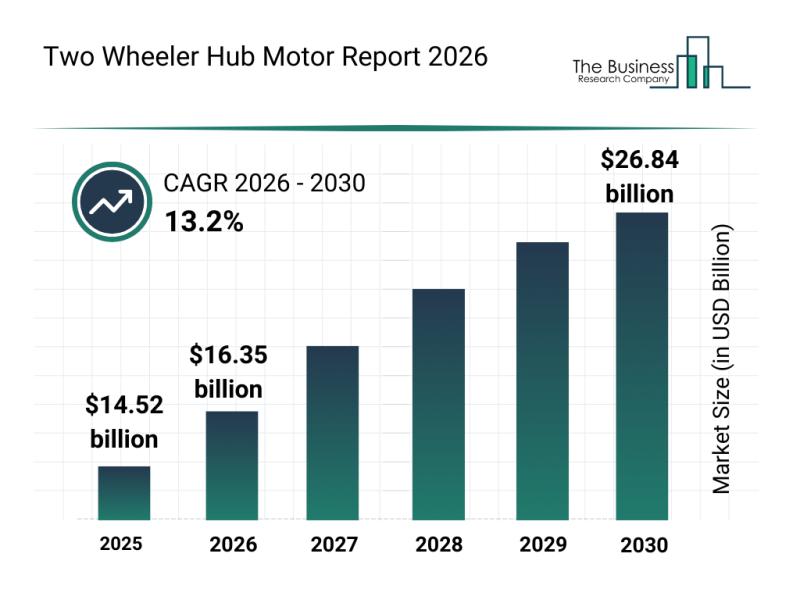
Competitive Analysis: Leading Companies and New Entrants in the Two Wheeler Hub …
The two wheeler hub motor market is set to experience significant expansion over the coming years, driven by a variety of technological advances and shifting consumer preferences. As electric mobility continues to gain traction globally, the demand for efficient and lightweight motor systems in two-wheelers is increasing. Here's an in-depth look at the market's size, major players, emerging trends, and key segments shaping its trajectory.
Projected Market Value and Growth Outlook…
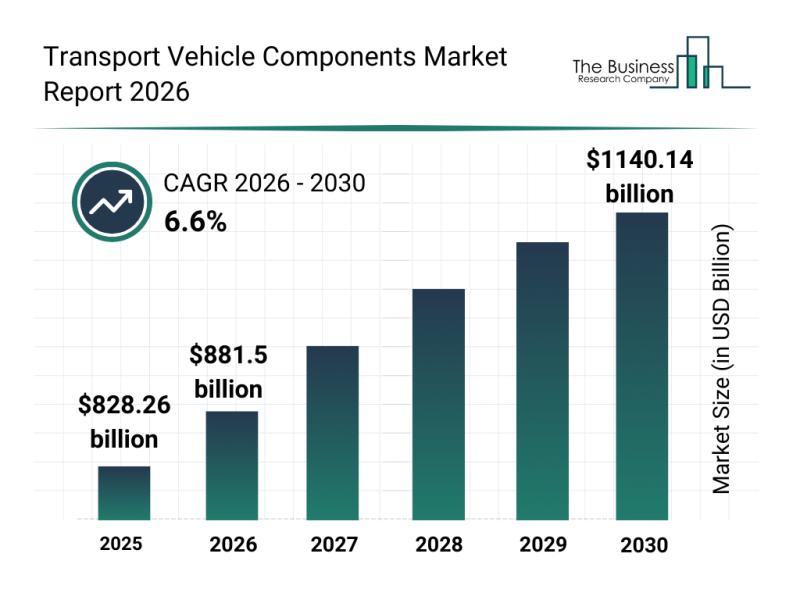
Segmentation, Major Trends, and Competitive Overview of the Transport Vehicle Co …
The transport vehicle components industry is on a strong growth trajectory, driven by rapid technological advancements and evolving market demands. As the shift toward electric and smart vehicles intensifies, this sector is set to expand considerably in the coming years. Let's explore the market size projections, key players, emerging trends, and segmentation details shaping this dynamic industry.
Projected Market Size Expansion of the Transport Vehicle Components Industry
The market for…
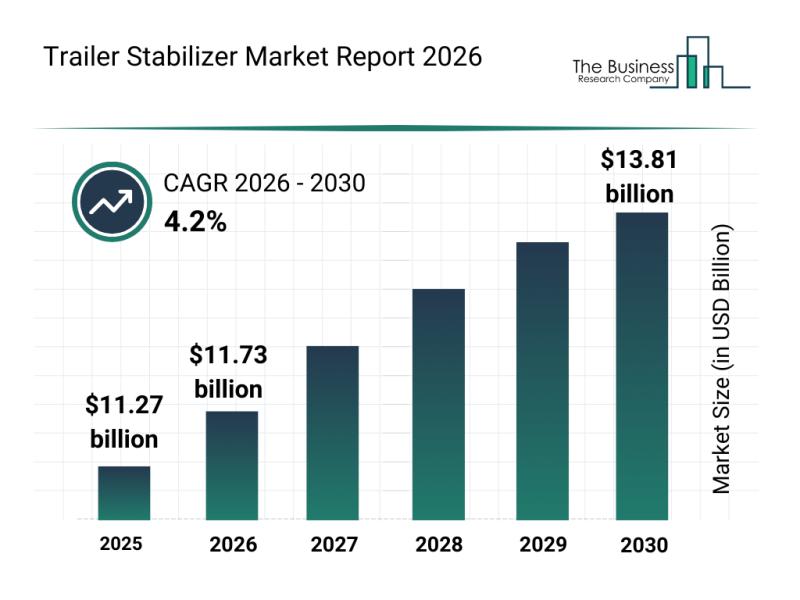
Market Trend Insights: The Impact of Recent Innovations on the Trailer Stabilize …
The trailer stabilizer market is positioned for consistent expansion over the coming years, driven by increasing demands for safer and more efficient trailer operations. As logistics and freight handling evolve, this market is adapting with innovative solutions that enhance safety and stability across various trailer types. Let's explore the size, leading players, trends, and segmentation of the trailer stabilizer industry for a clearer understanding of its future potential.
Forecasted Expansion and…
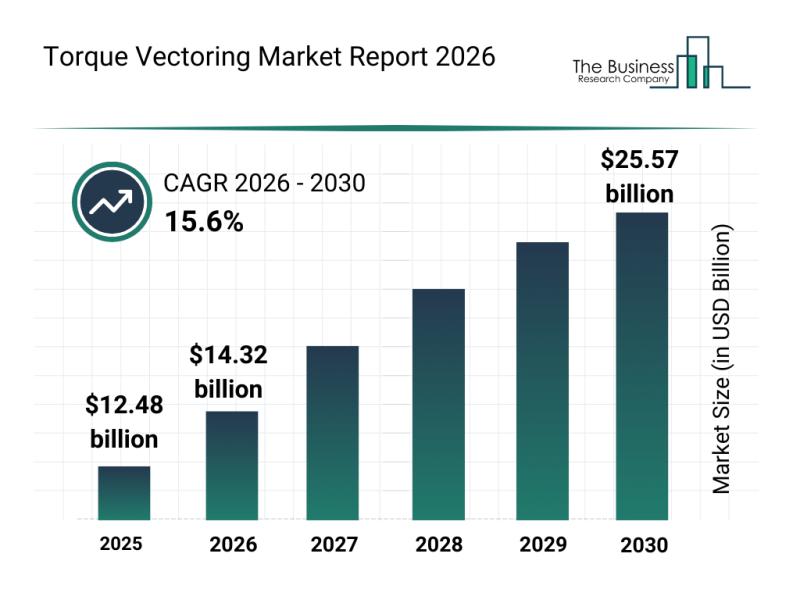
Torque Vectoring Market Overview, Key Trends, and Insights on Top Players
The torque vectoring market is on the brink of significant growth as advancements in automotive technology accelerate. This sector is becoming increasingly crucial due to shifts toward electric and hybrid vehicles, as well as greater demand for autonomous and connected driving features. Let's explore the market's size projections, influential factors, major players, emerging trends, and segmentation details to understand its dynamic landscape fully.
Projected Expansion and Market Size of the Torque…
More Releases for Reporting
ESG Reporting Software Market (2024-2034): Trends, Forecast, and Key Players Dri …
The ESG Reporting Software market is estimated to be valued at USD $888.2 million in 2024 and is expected to reach USD $3,752.6 million by 2034, growing at a compound annual growth rate (CAGR) of 15.5% from 2024 to 2034.
➤ The ESG Reporting Software study, published by Global Insight Services, assessed upcoming growth prospects and provided detailed information and useful statistics on market structure and size. For the forecast…
ESG Reporting Software Market Unlocking Sustainability Performance: A Market Ana …
ESG Reporting Software Market worth $2.25 Bn by 2031 - Exclusive Report by InsightAce Analytic Pvt. Ltd.
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global ESG Reporting Software Market- (By Component (Solutions, Services), By Deployment Type (On-premises, Cloud), By Organization Size (Large Enterprises, SMEs), By Vertical (BFSI, Government, Public Sector & Non-Profit, Retail)), Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
According to the…
Medication Error Reporting Market Strengthening Systems: How Medication Error Re …
Medication Error Reporting Market Assessment worth $ 1.32 Billion by 2030 - Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Medication Error Reporting Market- by Error Type (Knowledge-Based Error, Rule-Based Error, Action Based Error, and Memory Based Error), Occurrence (Hospitals, Community Pharmacies, and Multinational Retail Groups), Trends, Industry Competition Analysis, Revenue and Forecast To 2030."
Get a free sample…
Innovaccer Launches Its Web Interface Reporting Solution to Help Providers Overc …
Amid healthcare’s rapid transformation to value-based care, measuring the quality of care is crucial. However, the short time frame for reporting, the exhaustive list of patient records and the manual intensive reporting process can seem a bit overwhelming to the providers. Providers need a solution that simplifies the quality reporting process and leaves them with more time to actually focus on the quality of care.
Innovaccer Inc., the leading San Francisco-based…
Pharmacovigilance Market (Type Of Methods: Spontaneous Reporting, Intensified AD …
Intensifying regulatory expectations, tougher inspection system, and instant need for patient reporting boost the adoption rate of pharmacovigilance among pharmaceutical companies. Rise in the prevalence of acute and chronic diseases has consequently led to an increase in the incidences of drug consumption, thus leading to growth in the number of adverse drug events and drug toxicity cases. Furthermore, safety regulations, risk of high-profile safety issues, large volume of post-market events…
Arbor Reporting Portal
Arbor Financial Systems have just released a new feature, Arbor Reporting Portal (ARP). This portal can be used on all devices with browser access, and can be distributed to whomever, whenever and wherever. ARP allows traders and fund managers to create a wide range of reports, from simple grids to complex diagrams and analysis. This is the latest addition to Arbor’s array of technologies, having just published Arbor Mobile Reporting.
…
