Press release
The Strategic Role of Factory Audits in Global Manufacturing
Global supply chains are more interconnected and more vulnerable than ever before. Rising consumer expectations, evolving regulatory frameworks, and heightened scrutiny around environmental, social, and governance (ESG) practices have placed unprecedented pressure on manufacturers to demonstrate accountability. In this environment, factory audits have moved from being procedural exercises to becoming essential mechanisms of corporate governance.A factory audit is not a superficial compliance check, but a systematic, independent assessment of how a facility is managed and operated. It provides a holistic view of operational health, covering everything from equipment reliability and workforce training to quality management, health and safety standards, and ethical labor practices. For organizations dependent on complex supply networks, the insights generated from a factory audit directly influence procurement decisions, risk strategies, and long-term brand reputation.
Scope and Dimensions of a Comprehensive Factory Audit:
Modern factory audits are multi-dimensional evaluations, structured to assess both operational efficiency and broader compliance responsibilities. Each dimension reveals different aspects of a facility's capability and resilience.
• Quality Management Systems (QMS):
Audits examine the rigor of systems such as ISO 9001, with a focus on corrective action processes, documentation quality, traceability, and non-conformance management. Strong QMS frameworks underpin product reliability, ensuring that goods consistently meet contractual and regulatory standards. Weaknesses in this area often manifest as recurring defects, recalls, or supply chain disruptions.
• Production Capability and Processes:
Beyond machinery inspection, audits evaluate production capacity utilization, preventive maintenance schedules, workforce training levels, and workflow optimization. A facility's ability to meet demand without sacrificing quality depends on these interrelated factors. Gaps here can constrain scalability or create bottlenecks that compromise delivery timelines.
• Health, Safety, and Environmental (HSE) Compliance:
Compliance with occupational health regulations, environmental standards, and workplace safety protocols is central to legal risk management and employee welfare. Factory audits assess practices such as waste treatment, fire safety preparedness, and adherence to global benchmarks like ISO 14001. Non-compliance not only incurs regulatory penalties but increasingly attracts reputational damage in global markets.
• Social and Ethical Accountability:
Ethical sourcing has become a decisive factor for global investors and end consumers. Audits now include evaluation against frameworks such as SA8000, covering labor contracts, wage policies, working hours, and prohibitions on child or forced labor. Transparency in this domain is critical for maintaining market access and aligning with the ESG commitments of multinational buyers.
By covering these dimensions in a single structured process, audits generate an integrated assessment of a facility's operational reliability and compliance maturity.
Get Complete Details on Factory Audit: https://www.imarcgroup.com/services/factory-audit
Strategic Advantages of Factory Audits
When embedded into supply chain governance, factory audits provide enduring benefits that extend well beyond compliance. They enhance resilience, safeguard reputation, and support continuous operational improvement.
1. Risk Identification and Mitigation
• Operational Risk Control: Early detection of process inefficiencies, equipment vulnerabilities, or workforce skill gaps prevents escalation into costly disruptions.
• Regulatory Assurance: Independent evidence of compliance reduces exposure to legal penalties, customs delays, and trade restrictions.
• Supplier Due Diligence: For firms outsourcing production, factory audits verify whether suppliers possess the technical competence, ethical standards, and production capacity required for long-term reliability.
2. Driving Quality and Product Consistency
• Process Standardization: Audits promote harmonized practices across facilities, minimizing variation in product quality.
• Root Cause Diagnostics: Systematic identification of underlying issues - whether training deficits, equipment failure, or workflow errors - enables durable corrective action.
• Cultural Reinforcement: Repeated audits embed a culture of quality within organizations, aligning day-to-day practices with long-term brand commitments.
3. Operational Efficiency and Cost Optimization:
• Resource Optimization: Independent evaluation highlights opportunities to reduce energy intensity, streamline inventory systems, and enhance utilization of assets.
• Waste Reduction: Audits frequently identify avoidable inefficiencies in raw material usage, contributing to both cost savings and sustainability targets.
• Sustained Competitiveness: Efficiency gains translate into stronger market positioning, enabling firms to balance cost control with product differentiation.
Methodology: How Effective Audits Are Conducted:
The audit process is inherently collaborative. Skilled auditors engage with factory management and workforce teams to build a complete picture of operations. A structured methodology typically involves:
1. Pre-Audit Preparation: The process begins with a thorough review of existing documentation, including internal quality manuals, compliance records, training logs, and results from prior audits. This stage establishes a baseline understanding of the facility's current systems and performance. It also allows auditors to tailor their evaluation framework to the specific operational and regulatory context of the factory, ensuring that key risks are addressed systematically.
2. On-Site Evaluation: A central component of the audit is direct observation on the factory floor. Auditors assess the condition of production lines, the maintenance of machinery, the implementation of health and safety practices, and the overall alignment between documented policies and operational execution.
3. Stakeholder Interviews: To validate whether compliance frameworks are embedded into daily practice, auditors engage with employees across multiple organizational levels. Discussions with operators, supervisors, and senior managers reveal whether official policies are effectively communicated, understood, and consistently applied.
4. Analysis and Reporting: The findings are consolidated into a comprehensive, evidence-based report. Beyond cataloguing strengths and weaknesses, the report prioritizes recommendations based on risk exposure, feasibility, and potential impact. This allows management teams to allocate resources effectively and implement targeted corrective actions rather than pursuing generic improvement programs.
5. Follow-Up and Continuous Monitoring: Audits deliver the greatest value when they are part of an ongoing cycle. Periodic re-evaluations track whether corrective measures have been implemented successfully and whether improvements are sustainable over time. This continuous monitoring framework transforms audits from static assessments into dynamic mechanisms for long-term performance enhancement.
This structured approach ensures that audits are not one-off inspections but continuous improvement mechanisms that support operational excellence.
Strategic Outlook: Factory Audits as a Cornerstone of Supply Chain Assurance
The importance of factory audits has grown in parallel with shifts in global trade and manufacturing. Geopolitical realignments, diversification of sourcing strategies, and the rise of ESG-driven investment criteria have all elevated the role of independent verification in supply chains. For investors and operators, factory audits are no longer discretionary exercises but essential mechanisms of trust and assurance.
Evidence indicates that companies adopting systematic, research-driven audit programs achieve stronger long-term outcomes: reduced risk exposure, higher compliance scores, and measurable gains in efficiency. Beyond internal benefits, regular audits signal credibility to stakeholders: regulators, investors, and consumers, all of whom increasingly demand evidence of responsible operations.
As supply chains expand in scale and complexity, the role of audits will only deepen. They are not simply about verifying compliance but about building resilience, enabling transparency, and ensuring sustainable growth. For global manufacturers, factory audits have become integral to safeguarding competitiveness in an environment defined by both opportunity and scrutiny.
To explore IMARC Group's capabilities in factory audits, including methodology and sector-specific expertise, please see our service page.
How IMARC Can Help?
IMARC Group is a global management consulting firm that helps the world's most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
United States: +1-631-791-1145
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release The Strategic Role of Factory Audits in Global Manufacturing here
News-ID: 4171511 • Views: …
More Releases from IMARC Group
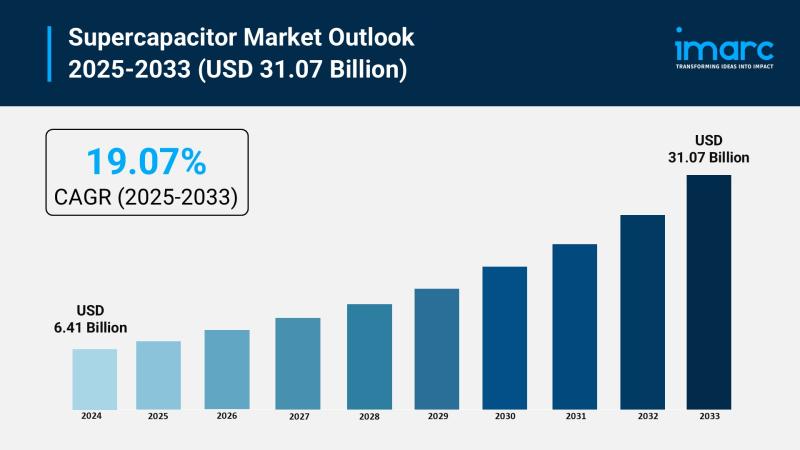
Supercapacitor Market Size to Reach $31.07B by 2033: Trends & Opportunities
Market Overview:
The supercapacitor market is experiencing rapid growth, driven by electrification of automotive systems, renewable energy and grid stabilization, and expansion of industrial automation and robotics. According to IMARC Group's latest research publication, "Supercapacitor Market Size, Share, Trends and Forecast by Product Type, Module Type, Material Type, End Use Industry, and Region, 2025-2033", the global supercapacitor market size was valued at USD 6.41 Billion in 2024. Looking forward, IMARC Group…
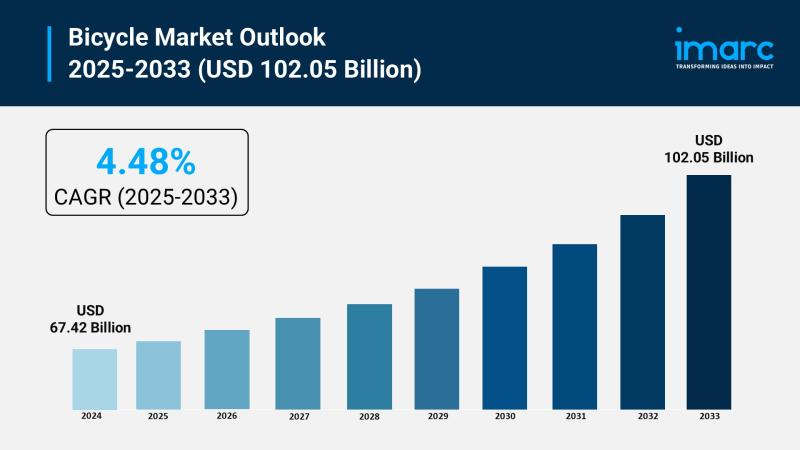
Bicycle Market Size to Surpass $102.05B by 2033: Growth & Insights
Market Overview:
The bicycle market is experiencing rapid growth, driven by global expansion of cycling infrastructure, rising health consciousness and preventative wellness, and technological advancements in e-bike propulsion. According to IMARC Group's latest research publication, "Bicycle Market Size, Share, Trends and Forecast by Type, Technology, Price, Distribution Channel, End User, and Region, 2025-2033", The global bicycle market size was valued at USD 67.42 Billion in 2024. Looking forward, IMARC Group estimates…
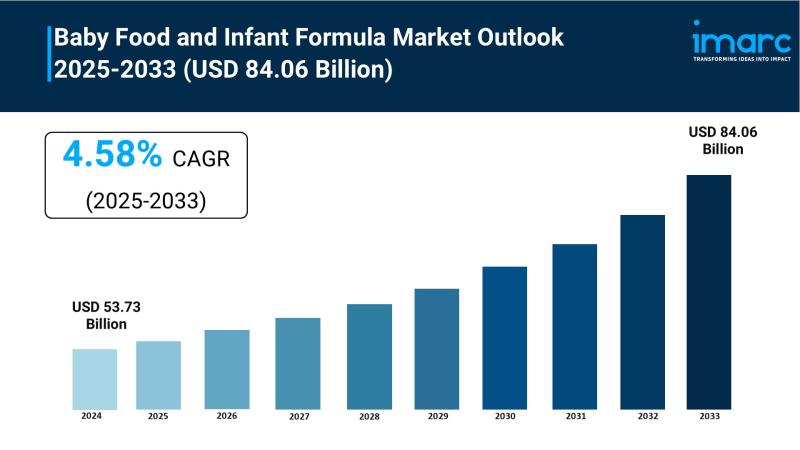
Baby Food and Infant Formula Market to Reach USD 84.06 Billion by 2033, Growing …
Market Overview:
The Baby Food and Infant Formula Market is experiencing steady expansion, driven by Increasing Awareness of Nutritional Needs for Infants, Rising Number of Working Women, and Technological Advancements and Product Innovation. According to IMARC Group's latest research publication, "Baby Food and Infant Formula Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2025-2033", The global baby food and infant formula market size reached USD 53.73 Billion in 2024.…
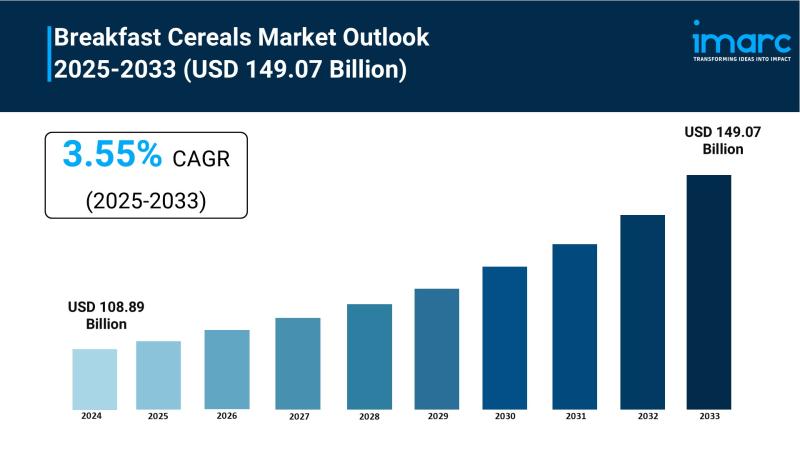
Breakfast Cereals Market to Reach USD 149.07 Billion by 2033, Growing at a CAGR …
Market Overview:
The Breakfast Cereals Market is experiencing rapid growth, driven by Health and Wellness Awareness, Busy Lifestyles and On-the-Go Demand and Rising Disposable Incomes and Global Market Expansion . According to IMARC Group's latest research publication, "Breakfast Cereals Market : Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2025-2033", The global breakfast cereals market size was valued at USD 108.89 Billion in 2024. Looking forward, IMARC Group estimates…
More Releases for Audits
Global Crypto Regulatory Updates Highlight Audits: Pepeto Audits Boosts Confiden …
Recent regulatory developments worldwide have a strong focus on transparency, audit verification, and platform accountability within the crypto industry. As governments and exchanges become more stringent on compliance measures, this time around presale projects are being evaluated as rigorously as ever.
This shift is sorting out low-quality launches and boosting structured ecosystems with proof of real infrastructure and audited smart contracts. Within this changing regulatory environment, the best crypto to…
Factory Audits: Purpose, Scope, Objectives and Benefits
What is a Factory Audit?
A factory audit is a thorough inspection of a manufacturing unit that checks if it complies with the quality benchmarks, operational requirements, and standards outlined.sets standards. It looks at multiple parameters, like production capacity, QC mechanisms, legal compliance, occupational health and safety, and social responsibility. Companies perform factory audits to confirm the reliability of their suppliers, reduce risks, and ensure uniformity in products. The audit also…
Factory Audits: Scope, Purpose, Types, Benefits
What is a Factory Audit?
A factory audit is an all-inclusive checkup of the manufacturing plant for it to align with quality, compliance, and operation standards. It evaluates such things as manufacturing ability, processes for quality controls, regulatory adherences, worker safety, and ethics. Factory audits are undertaken by businesses for authenticating vendor dependability, mitigating threats, and having consistency in product supply. The auditing procedure is important to ensure that equipment is…
AI in the Healthcare Audits Market Ensuring Trustworthy Care: The Rise of AI in …
AI in the Healthcare Audits Market to Record an Exponential CAGR by 2031 - Exclusive Report by InsightAce Analytic Pvt. Ltd.
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global AI in the Healthcare Audits Market- (By Audit Type (Pre-Billing Audits, Recovery Audits , Medicare, External Audits)), Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
According to the latest research by InsightAce Analytic, the Global…
ImmuneBytes Audits VIS's Swap Token
New Delhi, India- 26th April, 2022- A One-Stop Solution to All Your Security Problems
In order to strengthen any DeFi project's smart contract and detect faults, mistakes, and inefficiencies, ImmuneBytes performs several levels of verification and testing, both manual and automated, and static and dynamic.
With the Vis Swap Token smart contracts, our audit team performed thorough testing, starting with analyzing the code design patterns in which we reviewed the smart…
HIPAA 2016 Phase 2 Audits: Are You Ready?
HIPAA & HITECH now require additional security and privacy precautions to accommodate increased threats of digital hacking and hijacking with the use of all technologies. Adding (rather large) teeth to previous HIPAA rulings, stepped-up HIPAA audits are leading to disciplinary actions that involve serious consequences, including public embarrassment and significantly higher penalties. Clinic, small group and independent practitioners are especially vulnerable.
Dr. Marlene Maheu, a nationally renowned telehealth expert, will interview…
