Press release
Bispecific and Trispecific Antibodies Market Projected to Experience Significant Growth by 2035, Reports DelveInsight
Bispecific antibodies represent an innovative class of therapies designed to address complex diseases by simultaneously binding two targets with a single molecule. Most currently approved bispecifics are for oncology, particularly multiple myeloma and diffuse large B-cell lymphoma (DLBCL). Beyond oncology, only HEMLIBRA and VABYSMO are approved for conditions such as hemophilia A, wet age-related macular degeneration, and diabetic macular edema, while KIMMTRAK is approved for uveal melanoma. The pipeline remains strongest in multiple myeloma and non-small cell lung cancer (NSCLC), with increasing exploration in autoimmune disorders.The first approved bispecific, REMOVAB, targeted malignant ascites but was withdrawn in 2017. Key formats include BiTEs, DARTs, and TandAbs. Trispecific antibodies, though not yet approved, show promise by engaging three targets to boost efficacy and reduce resistance. Leading players include Johnson & Johnson Innovative Medicine, AbbVie/Genmab, Roche/Biogen, Amgen, Pfizer, and Akeso Biopharma, alongside emerging developers such as AstraZeneca, Regeneron, Merus, BioNTech, and Innovent Biologics. Collectively, bispecific and trispecific antibody markets are forecasted to expand significantly, driven by growing oncology demand.
(Albany, USA) DelveInsight has released its new study, "Bispecifics/Trispecifics Market Forecast and Competitive Landscape 2035," delivering a detailed assessment of the evolving bispecific and trispecific antibodies market. The report explores epidemiological patterns, market dynamics, and innovative treatment approaches, equipping stakeholders with critical knowledge to address challenges and leverage forthcoming opportunities in this rapidly advancing therapeutic area. Driven by research progress, growing awareness, and a robust drug pipeline, the bispecific/trispecific antibody market is expected to expand significantly through 2035.
The market is largely fueled by the demand for more targeted therapies across multiple cancers, including Acute Lymphocytic Leukemia (ALL), Diffuse Large B-cell Lymphoma (DLBCL), Follicular Lymphoma, Multiple Myeloma, Non-Small Cell Lung Cancer (NSCLC), and Small Cell Lung Cancer (SCLC). By simultaneously targeting multiple tumor mechanisms, these therapies not only improve clinical outcomes but also pave the way for more personalized cancer treatments, accelerating market growth within oncology by 2035.
Request a sample report @ Bispecifics/Trispecifics Market Forecast - https://www.delveinsight.com/report-store/bispecifics-trispecifics-competitive-landscape-and-market-forecast?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Highlights from the Bispecifics/Trispecifics Market Report include:
• In 2023, the oncology bispecific antibodies market across the 7MM was valued at approximately USD 690 million, with steady growth expected through 2035.
• The U.S. accounted for the largest concentration of bispecific antibody oncology indications in 2023, followed by the EU4 and the UK.
• Patient pools were largest for DLBCL and multiple myeloma, while acute lymphocytic leukemia-primarily affecting children-had comparatively smaller numbers.
• By 2035, DLBCL is anticipated to dominate the bispecific oncology market, reaching nearly USD 4.5 billion, with NSCLC and multiple myeloma following.
• ELREXFIO is expected to capture the leading share of the multiple myeloma bispecific antibody segment in the 7MM by 2035, approaching USD 1 billion.
• In March 2025, Sanofi and Dren Bio finalized an agreement under which Sanofi acquired DR-0201, a bispecific myeloid cell engager showing strong B-cell depletion in preclinical and early-stage trials.
• In November 2024, ZIIHERA received FDA approval for intravenous treatment in adults with previously treated, unresectable, or metastatic HER2-positive biliary tract cancer (BTC), making it the first dual HER2-targeted bispecific antibody cleared for HER2-positive BTC in the U.S.
• In September 2024, the FDA approved RYBREVANT combined with chemotherapy (carboplatin and pemetrexed) for adults with advanced or metastatic NSCLC harboring EGFR exon 19 deletions or L858R mutations, following progression on EGFR TKI therapy.
• Promising therapies under development include Ivonescimab, Zenocutuzumab, Petosemtamab, Cevostamab, Volrustomig, Rilvegostomig, Lutikizumab, PRV-3279, MEDI-7352, DR-0201, GB263T, CMG1A46, HPN328, among others.
• Major players advancing these therapies include AbbVie/Genmab, Roche/Biogen, Pfizer, Amgen, Akeso Biopharma, Summit Therapeutics, SystImmune, Biotheus, Alphamab, Sichuan Baili Pharmaceutical, Regeneron, Merus, Jazz Pharma/Zymeworks, Aurigene Oncology, and others.
For a detailed outlook on bispecifics/trispecifics market size, drug uptake, and epidemiological insights, click here: Bispecifics/Trispecifics Drugs Market - https://www.delveinsight.com/sample-request/bispecifics-trispecifics-competitive-landscape-and-market-forecast?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Overview of Bispecifics/Trispecifics
Bispecific and trispecific antibodies are an advanced class of immunotherapies designed to bind two or more distinct antigens at once. Unlike traditional monoclonal antibodies that act on a single antigen, these innovative molecules enhance specificity and therapeutic potential, particularly in oncology and immunology.
In cancer treatment, bispecifics have demonstrated strong anti-tumor activity by linking immune cells like T-cells or NK cells with tumor antigens, resulting in direct tumor destruction. Trispecifics advance this further by activating multiple immune pathways, reducing tumor resistance and improving outcomes.
Key pharmaceutical players, including Akeso, Summit Therapeutics, Roche, Merus, AstraZeneca, and Dren Bio, are at the forefront of developing these agents across cancers such as DLBCL, multiple myeloma, ALL, NSCLC, and SCLC. As clinical pipelines advance and more therapies receive approval, multispecific antibodies are positioned to redefine cancer care with precision-based strategies.
Download the report to explore how emerging therapies are adapting to patient needs: Bispecifics/Trispecifics Epidemiology Analysis - https://www.delveinsight.com/sample-request/bispecifics-trispecifics-competitive-landscape-and-market-forecast?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
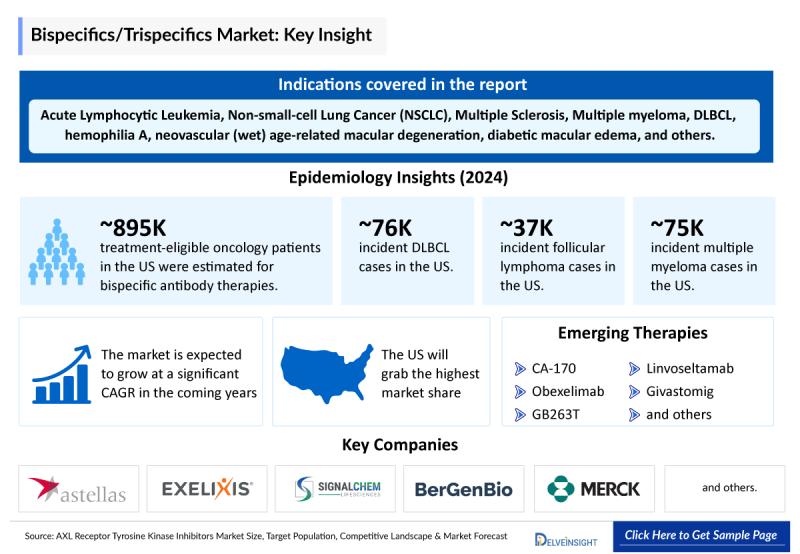
Bispecifics/Trispecifics Epidemiology Insights
The epidemiological analysis of bispecifics and trispecifics is segmented into three key categories: total cases across selected indications, the eligible patient pool, and treated cases within the 7MM (United States, EU4-Germany, France, Italy, Spain-along with the UK and Japan) from 2020 to 2035.
• In the U.S., the oncology treatment-eligible population for bispecific antibodies was estimated at around 895,600 patients. Additionally, the diagnosed prevalence of multiple sclerosis stood at 121,250 cases in 2024, with numbers expected to rise throughout the forecast period.
• Within the EU4 and the UK, Germany and France recorded the highest share of incident multiple myeloma cases in 2024, together accounting for about 24% of the total, whereas Spain reported the lowest. For multiple sclerosis, Germany had the largest diagnosed prevalence at roughly 35,250 cases, while France had the fewest.
• In Japan, multiple sclerosis demonstrated a gender disparity in 2024, with female patients (~700 cases) significantly outnumbering male patients (~350 cases).
Bispecific Antibodies vs. CAR-T Therapies
Although both CAR-T and bispecific antibodies are revolutionizing oncology, they differ in approach. CAR-T therapies, while effective in hematologic cancers, face hurdles in solid tumors due to their complex patient-specific manufacturing. Conversely, bispecifics offer off-the-shelf solutions with scalable production, showing durable responses in both solid and blood cancers. This gives them a competitive edge in accessibility and broader use.
Bispecifics/Trispecifics Drug Class Insights
• BLINCYTO (Amgen) marked a milestone in B-cell ALL treatment and continues to expand into first-line and chemo-free applications.
• RYBREVANT (J&J) is gaining ground in NSCLC and could rival TAGRISSO as clinical trials progress.
T-cell redirecting bispecifics (TRBAs) are rapidly advancing in hematology, targeting CD19, CD20, BCMA, CD123, and CD33, while solid tumor bispecifics are exploring antigen pairs like HER2/HER2, VEGF/Ang-2, and PD-1/CTLA-4.
For more details on therapies in development, visit: Bispecifics/Trispecifics Clinical Trials and Therapeutic Assessment - https://www.delveinsight.com/sample-request/bispecifics-trispecifics-competitive-landscape-and-market-forecast?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Bispecifics/Trispecifics Market Outlook
Bispecific antibodies utilize dual antigen-binding sites to simultaneously engage immune cells and tumor cells, while trispecific antibodies extend this mechanism to three targets. Although bispecifics have already secured multiple FDA approvals with over 500 clinical candidates in development, trispecifics remain in the early stages of research, with no late-phase trials or approvals to date.
Currently, seven bispecific antibodies are FDA-approved for blood cancers such as multiple myeloma, ALL, DLBCL, and follicular lymphoma. Meanwhile, trispecific research is expected to significantly expand the treatment landscape in the coming years.
Bispecifics/Trispecifics Market Drivers
• Advances in antibody engineering - Innovations in design and technology have improved efficacy, safety, and stability of bispecific and trispecific antibodies, expanding their therapeutic potential.
• High unmet medical need - These therapies address treatment gaps in relapsed or refractory cancers, offering new options where conventional treatments or CAR-T therapies may fail.
• Expanding applications - Strong clinical pipelines in hematologic malignancies (e.g., multiple myeloma, DLBCL) and growing exploration in solid tumors and autoimmune diseases.
• "Off-the-shelf" therapies - Compared to CAR-T, bispecific antibodies are easier to manufacture and scale, making them more accessible and commercially attractive.
• Rising oncology demand - Increasing cancer incidence and demand for targeted therapies drive rapid adoption and market expansion.
Bispecifics/Trispecifics Market Barriers
• Complex and costly production - Manufacturing bispecific and trispecific antibodies is challenging, leading to higher development and commercialization costs.
• Safety concerns - Risks such as cytokine release syndrome (CRS) and other immune-related toxicities pose hurdles for wider clinical adoption.
• Early-stage development of trispecifics - With no approved trispecific drugs yet, uncertainty remains around long-term safety, efficacy, and regulatory pathways.
• Competition from other modalities - CAR-T therapies, checkpoint inhibitors, and next-generation targeted treatments compete for the same patient populations.
• Market access and reimbursement challenges - High pricing may limit affordability and patient accessibility, especially in cost-sensitive markets.
Download a free sample report on bispecifics/trispecifics market forecast, size & share: https://www.delveinsight.com/report-store/bispecifics-trispecifics-competitive-landscape-and-market-forecast?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Report Scope
• Study Period: 2020-2035
• Coverage: 7MM (U.S., EU5, Japan)
• Key Companies: AbbVie/Genmab, Roche/Biogen, Pfizer, Amgen, Akeso Biopharma, Summit Therapeutics, Merus, Jazz Pharma/Zymeworks, SystImmune, Biotheus, Alphamab, Sichuan Baili Pharmaceutical, Regeneron, Aurigene Oncology, etc.
• Key Therapies: Ivonescimab, Zenocutuzumab, Petosemtamab, Cevostamab, Volrustomig, Rilvegostomig, Lutikizumab, PRV-3279, MEDI-7352, DR-0201, GB263T, CMG1A46, HPN328, among others.
• Analysis Includes: Market dynamics, SWOT/PESTLE analysis, Porter's Five Forces, BCG Matrix, market entry strategies, unmet needs, KOL/analyst insights, and reimbursement scenarios.
Contact Us:
Ankit Nigam
Manager Marketing
info@delveinsight.com
+14699457679
About DelveInsight
DelveInsight is a leading Life Science market research and business consulting company recognized for its off-the-shelf syndicated market research reports and customized solutions to firms in the healthcare sector.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Bispecific and Trispecific Antibodies Market Projected to Experience Significant Growth by 2035, Reports DelveInsight here
News-ID: 4170858 • Views: …
More Releases from DelveInsight Business Research

Biliary Tract Cancer Market: Growth Momentum Across 7MM to 2034 - DelveInsight
DelveInsight's "Biliary Tract Cancer Market Insights, Epidemiology, and Market Forecast-2034′′ report offers an in-depth understanding of the Biliary Tract Cancer, historical and forecasted epidemiology as well as the Biliary Tract Cancer market trends in the United States, EU4 (Germany, Spain, Italy, France) the United Kingdom and Japan.
To Know in detail about the Biliary Tract Cancer market outlook, drug uptake, treatment scenario and epidemiology trends, Click here; Biliary Tract Cancer…

Behcet's Disease Market: Expanding Revenue Landscape to 2034 - DelveInsight
DelveInsight's "Behcet's Disease Market Insights, Epidemiology, and Market Forecast-2034′′ report offers an in-depth understanding of the Behcet's Disease, historical and forecasted epidemiology as well as the Behcet's Disease market trends in the United States, EU4 (Germany, Spain, Italy, France) the United Kingdom and Japan.
To Know in detail about the Behcet's Disease market outlook, drug uptake, treatment scenario and epidemiology trends, Click here; Behcet's Disease Market Forecast
https://www.delveinsight.com/sample-request/behcets-disease-market?utm_source=openpr&utm_medium=pressrelease&utm_campaign=gpr
Some of the…

Anaphylaxis Market: Rapid Increment Driven by Innovation - DelveInsight
DelveInsight's "Anaphylaxis Market Insights, Epidemiology, and Market Forecast-2032′′ report offers an in-depth understanding of the Anaphylaxis, historical and forecasted epidemiology as well as the Anaphylaxis market trends in the United States, EU4 (Germany, Spain, Italy, France) the United Kingdom and Japan.
To Know in detail about the Anaphylaxis market outlook, drug uptake, treatment scenario and epidemiology trends, Click here; Anaphylaxis Market Forecast
https://www.delveinsight.com/sample-request/anaphylaxis-market?utm_source=openpr&utm_medium=pressrelease&utm_campaign=gpr
Some of the key facts of the Anaphylaxis…

Tendonitis Market: Strong Pharma Growth Forecast Through 2034 - DelveInsight
The Tendonitis market is expected to surge due to the disease's increasing prevalence and awareness during the forecast period. Furthermore, launching various multiple-stage Tendonitis pipeline products will significantly revolutionize the Tendonitis market dynamics.
DelveInsight's "Tendonitis Market Insights, Epidemiology, and Market Forecast-2034′′ report offers an in-depth understanding of the Tendonitis, historical and forecasted epidemiology as well as the Tendonitis market trends in the United States, EU5 (Germany, Spain, Italy, France,…
More Releases for Bispecific
Global Bispecific Antibody Market Size Bispecific Antibodies Clinical Trials FDA …
Global Bispecific Antibody Market, Drugs Sales, Patent, Price and Clinical Trials Insight 2029 Report Highlights:
• Bispecific Antibodies Development Proprietary Platforms Insight: > 30 Platforms
• Global Bispecific Antibodies Market Size Yearly and Quarterly Sales (2018 till 2023)
• Global Bispecific Antibodies Market Size 2023: > USD 8 Billion
• Global Bispecific Antibodies Market Forecast Till 2029
• Approved Bispecific Antibodies Yearly and Quarterly Sales (2018 till 2023)
• Approved Bispecific Antibodies Regional Sales (2018 till 2023)
• Clinical and Commercial Insight On Approved…
Bispecific Drug Innovation - Creative Biolabs Concludes Its Journey at the 15th …
On September 5, Creative Biolabs successfully concluded its participation in the 15th Annual World Bispecific Summit.
New York, USA - September 10, 2024 - The summit brought together leading experts in the bispecific antibody [https://www.creative-biolabs.com/bsab/bispecific-antibody-bsab-development-service.htm] (BsAb) field from around the globe, offering attendees a rich platform to discuss cutting-edge developments and future directions.
Image: https://www.getnews.info/uploads/b7ea13d648fcb7917ba9b55571b2ba35.png
In recent years, this field has grown rapidly, becoming a focal point in biopharmaceutical research and development. A…
Bispecific Antibody Drug Conjugates Development
The development of bispecific antibody drug conjugates (ADCs) marks a significant advancement in the field of targeted cancer therapy. These innovative molecules combine the specificity of bispecific antibodies with the powerful cytotoxic effects of drug conjugates, creating a new class of therapeutic agents that hold great promise for treating complex and resistant cancers. The process of developing these ADCs involves intricate design, engineering, and testing to ensure their safety, efficacy,…
Bispecific Antibodies: Revolutionizing Targeted Cancer Therapy
Bispecific antibodies (BsAbs) are revolutionizing targeted cancer therapy by offering a dual-targeting approach that enhances therapeutic efficacy and addresses tumor heterogeneity. These engineered antibodies are designed to recognize and bind two different antigens simultaneously, providing a more comprehensive attack on cancer cells and improving treatment outcomes.
One of the most successful examples of BsAbs is blinatumomab, which targets CD19 on B-cells and CD3 on T-cells. Blinatumomab has shown remarkable efficacy in…
Global Bispecific Antibody Market Research Report Forecast 2017 to 2021Global Bi …
Report Hive Market Research Released a New Research Report of 119 pages on Title " Global Bispecific Antibody Market Research Report Forecast 2017 to 2021 "with detailed Analysis, Forecast and Strategies.
The Global Bispecific Antibody Market Research Report Forecast 2017-2021 is a valuable source of insightful data for business strategists. It provides the Bispecific Antibody industry overview with growth analysis and historical & futuristic cost, revenue, demand and supply data (as…
Bispecific Antibodies Market - Global Industry Analysis 2024
Bispecific Antibodies Market Overview
Bispecific antibody (BsAb) is an artificial protein that is composed of fragments of two different monoclonal antibodies and has ability to bind to two different types of antigen. Cancer immunotherapy is the most widely explored application of bispecific antibody. Lung, breast and colon cancer are the wider applications of BsAb. Bispecific antibody simultaneously binds to a cytotoxic cell and target tumor cell and destroys it. Bispecific antibodies…