Press release
COVID-19 Treatment Landscape: FDA Approves Updated COMIRNATY LP.8.1 COVID-19 Vaccine | DelveInsight's Perspective on Market Impact, Patient Population and Pipeline Therapies
DelveInsight Business Research's latest analysis highlights the significant impact of the FDA's approval of Pfizer and BioNTech's updated COMIRNATY LP.8.1-adapted monovalent COVID-19 vaccine on August 26, 2025. This landmark approval marks a strategic shift toward risk-based vaccination strategies, targeting the most vulnerable populations while addressing the evolving SARS-CoV-2 landscape with improved variant-specific immunity against currently circulating sublineages.Key COVID-19 Market Highlights
*
COMIRNATY's LP.8.1 FDA approval represents a crucial advancement in pandemic preparedness, targeting the SARS-CoV-2 sublineage LP.8.1 with demonstrated improved immune responses against multiple circulating variants, including XFG, NB.1.8.1, and other contemporary sublineages.
*
COVID-19 affects millions globally, with continued circulation of evolving variants necessitating updated vaccine formulations to maintain protective immunity.
*
COVID-19 Companies: According to the Delveinsight's pipeline analysis, approximately 180+ companies are developing COVID-19 therapies, including Pfizer Inc. (NYSE: PFE), BioNTech SE (NASDAQ: BNTX), Moderna (NASDAQ: MRNA), Novavax (NASDAQ: NVAX), Ansun Pharm, Biophytis (NASDAQ: BPTS), GeneOne Life Science, Shionogi, among others, developing vaccines, antivirals, and therapeutic interventions.
*
The global COVID-19 therapeutic market continues to evolve with ongoing development of 200+ pipeline drugs across various stages of clinical development, addressing prevention, treatment, and management of COVID-19.
Market Impact and COVID-19 Patient Population
According to DelveInsight's analysis and the COVID-19 pipeline insight [https://www.delveinsight.com/report-store/covid-19-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=xpr], there remains significant medical need for effective COVID-19 prevention strategies. The LP.8.1-adapted vaccine addresses the evolving nature of SARS-CoV-2, where variants continue to emerge and circulate globally. The approval specifically targets adults aged 65 years and older and individuals ages 5-64 with at least one underlying condition that increases risk for severe COVID-19 outcomes.
The SARS-CoV-2 continues to cause substantial morbidity and mortality, with the virus primarily transmitted through respiratory droplets. COVID-19 causes respiratory illness with symptoms including cough, fever, and difficulty breathing. Elderly people and those with underlying medical conditions like cardiovascular disease, diabetes, chronic respiratory disease, and cancer are more likely to develop serious illness. The updated vaccine formulation represents a critical tool in protecting these high-risk populations during the upcoming respiratory season.
Furthermore, the COVID-19 report highlights that to date, 5 billion doses of Pfizer-BioNTech COVID-19 vaccine have been distributed globally, demonstrating the widespread utilization of mRNA technology in pandemic response. The LP.8.1-adapted formulation is expected to enhance protection against currently dominant viral strains, addressing vaccine effectiveness challenges as SARS-CoV-2 continues to evolve.
Download the COVID-19 Pipeline report to understand which other factors are driving the emerging vaccines @ COVID-19 Pipeline innovation [https://www.delveinsight.com/sample-request/covid-19-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=xpr].
COMIRNATY LP.8.1 Treatment Approach
COMIRNATY LP.8.1 represents an mRNA-based vaccine utilizing BioNTech's proprietary technology to target the most recent SARS-CoV-2 sublineage. Unlike previous formulations, this updated vaccine specifically targets the LP.8.1 variant, an offshoot of the JN.1 subvariant, providing enhanced immune recognition of currently circulating strains. The vaccine works by delivering mRNA instructions for producing the SARS-CoV-2 spike protein, enabling the immune system to recognize and respond to viral infection.
" This approval represents our continued commitment to providing updated COVID-19 vaccines that address evolving viral variants," said Pfizer leadership. "The LP.8.1-adapted formulation demonstrates improved immune responses against multiple sublineages, offering enhanced protection for vulnerable populations during the upcoming respiratory season. "
COMIRNATY Clinical Validation and Efficacy
The COMIRNATY LP.8.1 FDA approval was based on comprehensive clinical and preclinical evidence demonstrating improved immune responses against multiple circulating SARS-CoV-2 sublineages, including XFG, NB.1.8.1, and other contemporary variants, compared to previous JN.1- and KP.2-adapted formulations. Key clinical outcomes include enhanced neutralizing antibody responses and superior cross-reactivity against emerging variants. The vaccine maintains the established favorable safety profile supported by extensive real-world evidence from over 5 billion doses administered globally.
COVID-19 Competitive Landscape and Market Positioning
COMIRNATY LP.8.1 enters a highly competitive landscape that includes updated formulations from Moderna (Spikevax and mNEXSPIKE) and Novavax (Nuvaxoid), all targeting the same LP.8.1 variant lineage. The COVID-19 competitive landscape has evolved significantly since initial emergency use authorizations, with current management focusing on risk-based vaccination strategies rather than universal recommendations.
According to the Covid-19 pipeline analysis, the broader competitive ecosystem includes companies developing various therapeutic approaches: antiviral drugs (molnupiravir, paxlovid, remdesivir), anti-SARS-CoV-2 monoclonal antibodies, anti-inflammatory drugs, and emerging pipeline therapies. COMIRNATY's established safety profile, global distribution network, and proven mRNA platform provide competitive advantages in the evolving COVID-19 prevention landscape.
Explore the COVID-19 Drug Battle: COMIRNATY LP.8.1 COVID-19 Vaccine vs. other emerging therapies. Discover how these breakthrough Bronchiectasis therapies compare in efficacy, safety, cost, and market impact @ COVID-19 Vaccines Competitive Landscape [https://www.delveinsight.com/sample-request/covid-19-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=xpr].
Emerging COVID-19 Pipeline Therapies
DelveInsight's pipeline analysis reveals several companies actively developing next-generation COVID-19 therapies, including Ansun Pharm's DAS181 (Phase III recombinant sialidase enzyme), Biophytis' Sarconeos (BIO101) for COVID-19-related complications, GeneOne Life Science's GLS-5310 (DNA vaccine), and Shionogi's S-892216 for COVID-19-related acute respiratory distress syndrome. These pipeline candidates represent diverse approaches, including recombinant fusion proteins, small molecules, monoclonal antibodies, peptides, gene therapy, and novel delivery mechanisms.
Furthermore, the COVID-19 pipeline encompasses companies developing advanced antiviral therapies, immunomodulatory agents, and combination treatments. Despite this evolving landscape, mRNA vaccines like COMIRNATY maintain critical importance in pandemic preparedness through their ability to rapidly adapt to emerging variants and provide population-level immunity.
Broader COVID-19 Therapeutic Pipeline
Beyond vaccination, the pharmaceutical industry continues investigating COVID-19 therapeutics across multiple routes of administration (oral, intravenous, subcutaneous, parenteral, topical) and diverse molecule types. The pipeline analysis indicates ongoing development of treatments addressing acute infection, long COVID sequelae, and prevention strategies for immunocompromised populations.
Discover more COVID-19 pipeline therapies and the clinical development progress they are making @ COVID-19 Clinical Pipeline [https://www.delveinsight.com/sample-request/covid-19-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=xpr].
Industry Expert Perspective
Clinical experts emphasize the significance of updated vaccine formulations in maintaining pandemic preparedness. "The approval of LP.8.1-adapted vaccines represents a proactive public health strategy," commented leading infectious disease specialists. "By targeting currently dominant viral lineages, these updated formulations provide enhanced protection for vulnerable populations most at risk for severe COVID-19 outcomes."
Learn more about what other Industry experts are saying about COMIRNATY LP.8.1 COVID-19 Vaccine FDA Approval and how it will impact the COVID-19 treatment market @ Key Opinion Leaders on COVID-19 Pipeline. [https://www.delveinsight.com/sample-request/covid-19-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=xpr]
Looking Forward
The COMIRNATY LP.8.1 approval represents a paradigm shift toward risk-based COVID-19 vaccination strategies, focusing on populations most vulnerable to severe disease while maintaining flexibility to address viral evolution. DelveInsight's analysts emphasize that the transition from universal vaccination recommendations to targeted approaches reflects improved understanding of COVID-19 epidemiology and risk stratification.
As SARS-CoV-2 continues evolving, the success of variant-adapted vaccines like COMIRNATY LP.8.1 demonstrates the pharmaceutical industry's capacity for rapid platform adaptation and targeted intervention development. This approach may serve as a model for future pandemic preparedness strategies, combining established manufacturing capabilities with real-time variant surveillance to provide timely protection for high-risk populations. The availability of immediate shipping and distribution ensures rapid deployment ahead of the 2025-2026 respiratory season, potentially reducing COVID-19-related hospitalizations and severe outcomes in vulnerable populations.
Table of Contents
1. Key Insights
2. Report Introduction
3. COVID-19 Market Overview at a Glance
4. Methodology of COVID-19 Epidemiology and Market
5. Executive Summary of COVID-19
6. Key Events
7. Disease Background and Overview
8. COVID-19 Epidemiology and Patient Population
9. COVID-19 Patient Journey
10. COVID-19 Emerging Drugs
11. COVID-19: Market Analysis
12. KOL Views
13. SWOT Analysis
14. COVID-19 Unmet Needs
15. COVID-19 Market Access
16. Appendix
17. DelveInsight Capabilities
18. Disclaimer
19. About DelveInsight
About DelveInsight
DelveInsight is a leading market research and consulting firm specializing in disease-specific insights and therapeutic market analysis. Their reports integrate real-world data, clinical trial findings, and expert interviews to deliver comprehensive industry intelligence.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Arpit Anand
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=covid19-treatment-landscape-fda-approves-updated-comirnaty-lp81-covid19-vaccine-delveinsights-perspective-on-market-impact-patient-population-and-pipeline-therapies]
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: Nevada
Country: United States
Website: https://www.delveinsight.com/consulting/due-diligence-services
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release COVID-19 Treatment Landscape: FDA Approves Updated COMIRNATY LP.8.1 COVID-19 Vaccine | DelveInsight's Perspective on Market Impact, Patient Population and Pipeline Therapies here
News-ID: 4165747 • Views: …
More Releases from ABNewswire
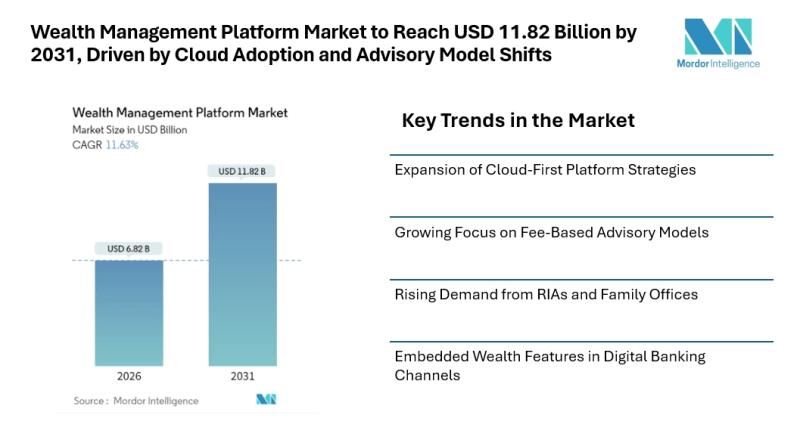
Wealth Management Platform Market to Reach USD 11.82 Billion by 2031, Driven by …
Mordor Intelligence has published a new report on the wealth management platform market, offering a comprehensive analysis of trends, growth drivers, and future projections.
Wealth Management Platform Market Overview
The wealth management platform market continues to gain steady attention as financial institutions modernize advisory operations and respond to changing investor expectations. According to Mordor Intelligence, the wealth management platform market size [https://www.mordorintelligence.com/industry-reports/wealth-management-platform-market?utm_source=abnewswire] stood at USD 6.82 billion in 2026 and is projected…

Why UK Taxpayers Are Choosing the Best Self Assessment Software in 2026
As HMRC continues to support online filing, self assessment software has become an essential tool rather than an optional one. The best platforms help users stay organised throughout the year, not just at deadline time. Pie's approach reflects this shift, focusing on simplicity, trust and transparency, while reinforcing its core message: "It's your money. Claim it."
LONDON, United Kingdom - As self assessment deadlines approach and digital filing becomes the default,…

Beycome Secures $2.5 Million Seed Funding Round to Scale Digital Real Estate Pla …
Image: https://www.abnewswire.com/upload/2026/02/01902a4178e53eaeed8cf0351beeed89.jpg
Beycome [https://www.beycome.com/], a tech-first, direct-to-consumer real estate platform, announced today the closing of a $2.5 million seed funding round. InsurTech Fund led the oversubscribed round with participation from Pivot Ventures, Florida Opportunity Fund, RedShift Capital, Neer Venture Capital, Kima Ventures, Ignite Venture, and Founders Future, alongside several strategic angel investors.
Founded in 2020, Beycome provides a digital ecosystem that allows homeowners and buyers to conduct transactions without traditional percentage-based commissions.…

Montgomery Roofing - Lorena Roofers Enhances Roofing Maintenance Options for Hom …
Montgomery Roofing - Lorena Roofers continues to support homeowners and businesses in Lorena and nearby areas with dependable, locally focused roofing care. With an emphasis on consistent service, clear communication, and practical solutions, Montgomery Roofing - Lorena Roofers remains dedicated to protecting properties and meeting the ongoing needs of the communities it serves.
Montgomery Roofing Lorena Roofers continues to strengthen its local presence by improving access to dependable roofing maintenance [https://roofstexas.com/lorena-roofers/#:~:text=bitumen%0A%E2%80%93%20EPDM-,Roofing%20Maintenance,-Services]…
More Releases for COVID
COVID-19 Diagnostics Market Analysis and Forecast to 2033: COVID-19 and Post-COV …
The COVID-19 diagnostics market is projected to reach a size of US$ 79.41 billion in 2023, with a robust compound annual growth rate (CAGR) of 7.87% from 2023 to 2033. This growth is primarily driven by technological advancements in diagnostic tools and methodologies, enabling faster and more accurate detection of the virus. However, the market faces several challenges, including the perception of high costs, along with concerns regarding test accuracy…
COVID-19 Molecular Diagnostics Market Insights by 2027 & Covid-19 Analysis | BD, …
This detailed assessment COVID-19 Molecular Diagnostics market report highlights data about various aspects, which includes growth factors and restraints. Crucial information about market scenario provided in this market report greatly helps key stakeholder in making right decision before making an investment in the market. This report further provides an overview on well-known industries, their market contribution, successful market strategies and latest advancements in present contexts. It also covers market analysis…
Europe COVID-19 Diagnostic Market Size | COVID-19 Impact Analysis | Forecast to …
Europe COVID-19 Diagnostic market is estimated to reach $2.6 billion in 2027, by growing at a CAGR of -22.9 % during the forecast period (2021-2027). Several projects for the development of COVID-19, diagnosis, and treatment were reported over the last few months. Additionally, on 22 July 2020, the European Commission declared nearly $117.8 million to co-fund a call for supporting R&D of coronavirus vaccines, and to develop diagnostic tests, treatments,…
Anti-Covid nasal spray Market (Covid-19 Impact)| Game Changer For Globe | SaNOti …
Global Anti-Covid nasal spray Market Size, Status and Forecast 2026
Israel and New Zealand have given interim approval for the sale of biotech firm SaNOtize Research and Development’s Nitric Oxide Nasal Spray (NONS) which could help prevent transmission of the COVID-19 virus, the company said on Monday. (Reuters)
A self-administered nitric oxide nasal spray (NONS) made by Vancouver-based biotech firm SaNOtize has been found to dramatically reduce Covid-19 viral load in infected…
COVID-19 Imparts Positive Impact on Covid-19 Treatment Market | 2020-2027
The Covid-19 treatment market is projected to grow due to increasing cases worldwide requiring short- and long-term respiratory support and multiple partnerships for the development of treatments with clinical trials underway. However, governments all over the world are now responding to the threat of COVID-19 with all the essential measures such as social distancing, nationwide lockdown, travel restrictions, and large-scale quarantines that are anticipated to impact the businesses and consumer…
Industrial AI Computers Market 2020 - Pre-COVID-19 and Post-COVID-19 Comparison
Industrial AI Computers Industry 2020 Market Research Report" A new report added by DeepResearchReports.com to its research database. Industrial AI Computers Market is segmented by Regions/Countries. All the key market aspects that influence the Industrial AI Computers market currently and will have an impact on it have been assessed and propounded in the Industrial AI Computers market research status and development trends reviewed in the new report.
Download Free PDF Sample…
