Press release
Spinocerebellar Ataxias Clinical Trials, Companies, Therapeutic Assessment, Therapies, Treatment Algorithm, Pipeline | Biohaven Pharmaceuticals, Vico Therapeutics B. V., Steminent Biotherapeutics
DelveInsight's, "Spinocerebellar Ataxias - Pipeline Insight, 2025" report provides comprehensive insights about 8+ companies and 10+ pipeline drugs in Spinocerebellar Ataxias pipeline landscape. It covers the pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.DelveInsight's analysis highlights that over 8 key companies are actively engaged in developing more than 10 treatment therapies for Spinocerebellar Ataxias.
Spinocerebellar Ataxias Overview:
Ataxia is characterized by a loss of voluntary muscle coordination, resulting in impaired control of movement, gait instability, abnormal eye movements, and speech difficulties. Spinocerebellar ataxia (SCA) is a rare, progressive, inherited neurodegenerative disorder with an autosomal dominant inheritance pattern that primarily affects the cerebellum. It is part of the broader group of hereditary cerebellar ataxias. To date, more than 40 genetically distinct types of SCA have been identified, classified by their genetic loci in order of discovery, with SCA1 being the first described subtype.
The exact disease mechanisms are not fully understood, but several pathways are implicated. Genetic mutations may cause abnormal protein production, transcriptional dysregulation, defective autophagy, ion channel dysfunction, mitochondrial impairment, and toxic RNA gain-of-function effects. Abnormal proteins, such as ataxins, are usually degraded by the ubiquitin-proteasome system; however, they may form aggregates instead. These mutant ataxins also interact abnormally with other proteins like the TATA-binding transcription factor and CREB-binding protein, leading to disrupted transcription and unregulated gene expression.
Many SCAs demonstrate anticipation, where trinucleotide repeat expansions increase with each generation, worsening disease onset and severity. Expanded CAG repeats are characteristic of SCA1, 2, 3, 6, 7, 8, 12, and 17. Other subtypes involve different repeat expansions, such as ATTCT in SCA10, TGGAA in SCA31, GGCCTG in SCA36, and ATTTT in SCA37. Additionally, some SCAs are linked to single nucleotide variants or structural genomic changes-for example, SCA5, 13, 14, and 19 arise from missense mutations, while SCA15, 20, and 39 are associated with gene deletions or duplications.
Currently, there is no cure for SCA, and treatment focuses on symptom management. Supportive therapies include antiepileptic drugs for seizures, botulinum toxin for dystonia, beta-blockers or primidone for tremors, antidepressants for mood disorders, and levodopa for parkinsonism. These interventions aim to improve quality of life by addressing complications rather than halting disease progression.
Request for a detailed insights report on Spinocerebellar Ataxias pipeline insights [https://www.delveinsight.com/report-store/spinocerebellar-ataxias-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
"Spinocerebellar Ataxias Pipeline Insight 2025" report by DelveInsight provides a comprehensive analysis of the ongoing clinical development activities and growth prospects across the Spinocerebellar Ataxias Therapeutics Market.
Key Takeaways from the Spinocerebellar Ataxias Pipeline Report
*
DelveInsight's Spinocerebellar Ataxias (SCA) pipeline report highlights a strong therapeutic landscape, with 8+ companies actively developing over 10 potential treatment candidates.
*
Leading players such as Biohaven Pharmaceuticals, Vico Therapeutics B.V., Steminent Biotherapeutics, and others are advancing novel therapies aimed at improving outcomes for patients with SCA.
*
Promising candidates in development include Troriluzole, VO659, and several others in different clinical stages.
*
In the fourth quarter of 2024, Biohaven submitted a New Drug Application (NDA) to the U.S. FDA for troriluzole, seeking approval across all SCA genotypes. The application qualifies for priority review, supported by the FDA's prior orphan drug and fast-track designations for the therapy.
Spinocerebellar Ataxias Pipeline Analysis
The report provides insights into:
*
The report provides detailed insights into the key companies that are developing therapies in the Spinocerebellar Ataxias Market.
*
The report also evaluates different therapeutic candidates segmented into early-stage, mid-stage, and late-stage of development for Spinocerebellar Ataxias treatment.
*
It analyzes the key companies involved in targeted therapeutics development with respective active and inactive (dormant or discontinued) projects.
*
It navigates the emerging drugs under development based on the stage of development, route of administration, target receptor, monotherapy or combination therapy, a different mechanism of action, and molecular type.
*
Detailed analysis of collaborations (company-company collaborations and company-academia collaborations), licensing agreement, and financing details for future advancement of the Spinocerebellar Ataxias market.
Download our free sample page report on Spinocerebellar Ataxias pipeline insights [https://www.delveinsight.com/sample-request/spinocerebellar-ataxias-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
Spinocerebellar Ataxias Emerging Drugs
*
Troriluzole: Biohaven Pharmaceuticals
*
VO659: Vico Therapeutics B. V.
Spinocerebellar Ataxias Companies
There are over eight major companies actively developing therapies for Spinocerebellar Ataxias. Among them, Biohaven Pharmaceuticals has the most advanced drug candidates, currently in Phase III clinical trials.
DelveInsight's report covers around 10+ products under different phases of clinical development like
*
Late stage products (Phase III)
*
Mid-stage products (Phase II)
*
Early-stage product (Phase I) along with the details of
*
Pre-clinical and Discovery stage candidates
*
Discontinued & Inactive candidates
Spinocerebellar Ataxias pipeline report provides the therapeutic assessment of the pipeline drugs by the Route of Administration. Products have been categorized under various ROAs such as
*
Intravenous
*
Subcutaneous
*
Oral
*
Intramuscular
Spinocerebellar Ataxias Products have been categorized under various Molecule types such as
*
Monoclonal antibody
*
Small molecule
*
Peptide
Download Sample Pages to Get an in-depth Assessment of the Emerging Spinocerebellar Ataxias Therapies and Key Companies: Spinocerebellar Ataxias Clinical Trials and advancements [https://www.delveinsight.com/report-store/spinocerebellar-ataxias-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
Spinocerebellar Ataxias Pipeline Therapeutic Assessment
- Spinocerebellar Ataxias Assessment by Product Type
- Spinocerebellar Ataxias By Stage
- Spinocerebellar Ataxias Assessment by Route of Administration
- Spinocerebellar Ataxias Assessment by Molecule Type
Download Spinocerebellar Ataxias Sample report to know in detail about the Spinocerebellar Ataxias treatment market @ Spinocerebellar Ataxias Therapeutic Assessment [https://www.delveinsight.com/sample-request/spinocerebellar-ataxias-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
Table of Content
1. Report Introduction
2. Executive Summary
3. Spinocerebellar Ataxias Current Treatment Patterns
4. Spinocerebellar Ataxias - DelveInsight's Analytical Perspective
5. Therapeutic Assessment
6. Spinocerebellar Ataxias Late-Stage Products (Phase-III)
7. Spinocerebellar Ataxias Mid-Stage Products (Phase-II)
8. Early Stage Products (Phase-I)
9. Pre-clinical Products and Discovery Stage Products
10. Inactive Products
11. Dormant Products
12. Spinocerebellar Ataxias Discontinued Products
13. Spinocerebellar Ataxias Product Profiles
14. Spinocerebellar Ataxias Key Companies
15. Spinocerebellar Ataxias Key Products
16. Dormant and Discontinued Products
17. Spinocerebellar Ataxias Unmet Needs
18. Spinocerebellar Ataxias Future Perspectives
19. Spinocerebellar Ataxias Analyst Review
20. Appendix
21. Report Methodology
Request the Sample PDF to Get Detailed Insights About the Spinocerebellar Ataxias Pipeline Reports Offerings [https://www.delveinsight.com/report-store/spinocerebellar-ataxias-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports Pharma companies by providing comprehensive end-to-end solutions to improve their performance.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Kritika Rehani
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=spinocerebellar-ataxias-clinical-trials-companies-therapeutic-assessment-therapies-treatment-algorithm-pipeline-biohaven-pharmaceuticals-vico-therapeutics-b-v-steminent-biotherapeutics]
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: Nevada
Country: United States
Website: https://www.delveinsight.com/
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Spinocerebellar Ataxias Clinical Trials, Companies, Therapeutic Assessment, Therapies, Treatment Algorithm, Pipeline | Biohaven Pharmaceuticals, Vico Therapeutics B. V., Steminent Biotherapeutics here
News-ID: 4160471 • Views: …
More Releases from ABNewswire

Innovative Connections Adds Six New Experts To The Team
FORT COLLINS, Colo. - Dec. 17, 2025 - Innovative Connections [https://innovativeconnectionsinc.com/], a leadership consulting firm dedicated to transforming organizations through connection, purpose, and courageous leadership, has added six new professionals to the team.
The six new consultants, JoAnn Auger, John P. Broschak, Tim Smith, Donna Pearring, JoEllen Wilkins and Debby Neely, bring years of experience from small businesses, Fortune 500s, construction, insurance, financial institutions and engineering.
*
JoAnn Auger: JoAnn brings over 30…
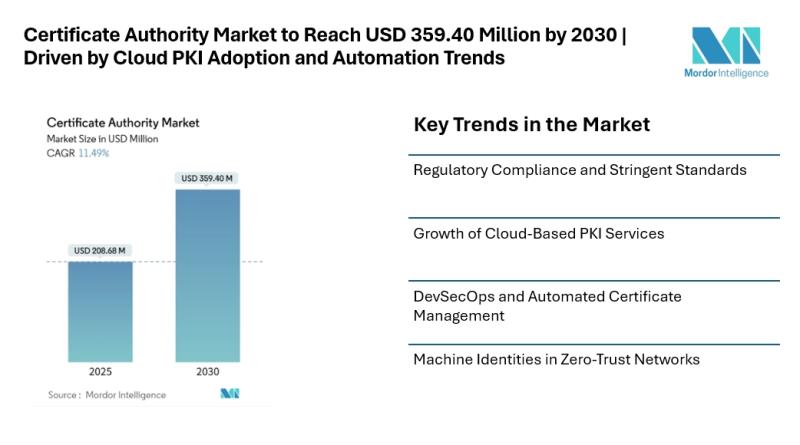
Certificate Authority Market to Reach USD 359.40 Million by 2030 | Driven by Clo …
Mordor Intelligence has published a new report on the Certificate Authority Market, offering a comprehensive analysis of trends, growth drivers, and future projections.
Certificate Authority Market Overview
The certificate authority market [https://www.mordorintelligence.com/industry-reports/certificate-authority-market?utm_source=abnewswire] is poised for significant growth over the next few years, with an estimated market size of USD 208.68 million in 2025 and a forecasted value of USD 359.40 million by 2030, reflecting a CAGR of 11.49%. This growth is primarily…

Copenhagen Emerges as a Popular Destination for Secret and Surprise Engagement P …
Copenhagen is seeing increased interest from international couples planning secret and surprise engagement proposals. This release features Copenhagen photographer Aleks Jakobsons, who photographs dozens of proposals each year, and outlines why couples choose the city for its atmosphere, accessibility, and personalised proposal planning supported by local expertise.
Copenhagen, Denmark - December 17, 2025 - Copenhagen is increasingly on the radar of couples who want to combine travel with one of the…
DermaStation Skin Clinic leverages superior-grade laser technology for enhanced …
The popular Delhi-based skin clinic aims to make pigmentation treatment highly safe and effective.
In a bid to ensure better and surprising results to its clients, DermaStation, a leading skin clinic in Delhi leverages advanced laser technologies for focused pigmentation treatment in Delhi [https://www.dermastation.com/pigmentation+treatment+in+delhi+hyperpigmentation+treatment+delhi.html].
The expert dermatologists at DermaStation by leveraging the all-new highly-innovative laser dermatology suite, aims to deliver better results to clients struggling with mild to hyper pigmentation.
Speaking about the…
More Releases for Spinocerebellar
Spinocerebellar Ataxia (SCA) Market to Reach USD 2.1 Billion by 2034
Spinocerebellar ataxia (SCA) refers to a group of rare, inherited neurodegenerative disorders characterized by progressive loss of coordination, balance, and motor control due to cerebellar and spinal cord dysfunction. With more than 40 genetic subtypes identified (SCA1-SCA48), these disorders are typically autosomal dominant and present in adolescence or adulthood. Symptoms worsen over time, leading to gait instability, slurred speech, tremors, and, in advanced cases, severe disability.
Download Full PDF Sample Copy…
Spinocerebellar Ataxias (SCA) Market reaching approximately USD 2.6 billion by 2 …
Spinocerebellar ataxias (SCAs) are a group of rare, inherited neurodegenerative disorders characterized by progressive problems with movement, balance, and coordination. Caused by genetic mutations that affect the cerebellum and sometimes other parts of the nervous system, SCAs are classified into multiple subtypes (such as SCA1, SCA2, SCA3, and others), each with distinct genetic and clinical features.
Patients often experience gait instability, slurred speech, vision problems, and difficulty with fine motor skills,…
Spinocerebellar Ataxia Market Analysis: Opportunities & Forecast 2024-2031
Spinocerebellar Ataxia Market report, published by DataM Intelligence, provides in-depth insights and analysis on key market trends, growth opportunities, and emerging challenges. Committed to delivering actionable intelligence, DataM Intelligence empowers businesses to make informed decisions and stay ahead of the competition. Through a combination of qualitative and quantitative research methods, it offers comprehensive reports that help clients navigate complex market landscapes, drive strategic growth, and seize new opportunities in an…
Spinocerebellar Ataxias Market Value Strategic Analysis | Biohaven Pharmaceutica …
According to HTF Market Intelligence, the Spinocerebellar Ataxias market to witness a CAGR of xx% during the forecast period (2024-2030). The Latest published a market study on Global Spinocerebellar Ataxias Market provides an overview of the current market dynamics in the Global Spinocerebellar Ataxias space, as well as what our survey respondents- all outsourcing decision-makers- predict the market will look like in 2030. The study breaks the market by revenue…
G7 Spinocerebellar Ataxia Market 2024 Projections, Trends and Forecast 2024 - Da …
A new Report by DataM Intelligence, titled "G7 Spinocerebellar Ataxia Market: Industry Trends, Share, Size, Growth, Opportunity and Forecast 2024-2031,"" offers a comprehensive analysis of the industry, which comprises insights on the G7 Spinocerebellar Ataxia market analysis. The report also includes competitor and regional analysis, and contemporary advancements in the market.
This report has a complete table of contents, figures, tables, and charts, as well as insightful analysis. The G7 Spinocerebellar…
Spinocerebellar Ataxia Market to Witness Growth by 2032, Estimates DelveInsight
DelveInsight's "Spinocerebellar Ataxia Market Insights, Epidemiology, and Market Forecast-2032" report delivers an in-depth understanding of Spinocerebellar Ataxia, historical and forecasted epidemiology as well as the Spinocerebellar Ataxia market trends in the United States, EU5 (Germany, Spain, Italy, France, and United Kingdom), and Japan.
The Spinocerebellar Ataxia market report provides current treatment practices, emerging drugs, the market share of the individual therapies, and the current and forecasted Spinocerebellar Ataxia market size…
