Press release
Acetaminophen/Hydrocodone (Vicodin) Manufacturing Plant Report 2025 | Utility requirements and Cost Involved
Acetaminophen/Hydrocodone combination is a prescription pharmaceutical product combining acetaminophen (a non-opioid analgesic) with hydrocodone (an opioid analgesic) used for managing moderate to severe pain. This controlled substance requires strict manufacturing protocols, quality assurance measures, and regulatory compliance due to its opioid component and potential for abuse. The combination provides enhanced pain relief while reducing the required opioid dosage, making it a widely prescribed medication in pain management therapy under strict medical supervision.Setting up an acetaminophen/hydrocodone manufacturing plant requires specialized pharmaceutical equipment including API synthesis units, tablet compression machines, coating systems, quality control laboratories, secure storage facilities, and waste management systems. Critical considerations include location compliance with DEA regulations, adherence to Good Manufacturing Practices (GMP), obtaining Schedule II drug manufacturing licenses, and implementing robust security and tracking systems for controlled substances.
IMARC Group's report, titled "Acetaminophen/Hydrocodone Manufacturing Plant Project Report 2025: Industry Trends, Plant Setup, Machinery, Raw Materials, Investment Opportunities, Cost and Revenue," provides a complete roadmap for setting up an acetaminophen/hydrocodone manufacturing plant. It covers a comprehensive market overview to micro-level information such as unit operations involved, raw material requirements, utility requirements, infrastructure requirements, machinery and technology requirements, manpower requirements, packaging requirements, transportation requirements, etc.
Request for a Sample Report: https://www.imarcgroup.com/acetaminophen-hydrocodone-manufacturing-plant-project-report/requestsample
Acetaminophen/Hydrocodone Industry Outlook 2025:
The acetaminophen/hydrocodone industry outlook for 2025 shows controlled demand driven by legitimate chronic pain management needs and an aging population requiring long-term pain relief solutions. Regulatory scrutiny continues to intensify due to opioid crisis concerns, leading to stricter prescribing guidelines, enhanced monitoring systems, and abuse-deterrent formulation requirements.
Market dynamics are influenced by generic competition, prescription monitoring programs, and alternative pain management therapies. Manufacturers are investing heavily in advanced security systems, tracking technologies, and abuse-deterrent formulations to comply with evolving regulations. Despite regulatory challenges and market constraints, the legitimate medical need for effective pain management ensures continued but carefully controlled market demand in 2025.
Key Insights for Acetaminophen/Hydrocodone Manufacturing Plant Setup:
Detailed Process Flow
• Product Overview
• Unit Operations Involved
• Mass Balance and Raw Material Requirements
• Quality Assurance Criteria
• Technical Tests
• Controlled Substance Protocols
• Security and Tracking Systems
Project Details, Requirements and Costs Involved
• Land, Location and Site Development
• Plant Layout and Security Infrastructure
• Machinery Requirements and Costs
• Raw Material Requirements and Costs
• Packaging Requirements and Costs
• Transportation Requirements and Costs
• Utility Requirements and Costs
• Human Resource Requirements and Costs
• Regulatory Compliance Costs
Capital Expenditure (CapEx) and Operational Expenditure (OpEx) Analysis
Project Economics
• Capital Investments
• Operating Costs
• Expenditure Projections
• Revenue Projections
• Taxation and Depreciation
• Profit Projections
• Financial Analysis
• Regulatory Cost Impact
Profitability Analysis
• Total Income
• Total Expenditure
• Gross Profit
• Gross Margin
• Net Profit
• Net Margin
Request for Customized Report: https://www.imarcgroup.com/request?type=report&id=12450&flag=E
Key Cost Components of Setting Up an Acetaminophen/Hydrocodone Manufacturing Plant:
• Land & Secure Infrastructure -- Specialized pharmaceutical facilities with enhanced security perimeters, controlled access zones, vault storage for controlled substances, and DEA-compliant construction
• Raw Material Procurement -- High-grade acetaminophen, hydrocodone API with strict chain of custody, pharmaceutical excipients, and tamper-evident packaging materials
• Machinery & Equipment -- cGMP-compliant synthesis reactors, tablet compression systems, coating machines, serialization equipment, and advanced analytical instruments
• Labor & Specialized Workforce -- Licensed pharmacists, quality assurance specialists, security personnel, regulatory affairs experts, and GMP-trained manufacturing operators
• Energy & Utilities -- Clean room HVAC systems, purified water generation, compressed air, backup power systems, and environmental monitoring
• Quality Control & Testing -- Advanced analytical laboratories for potency testing, dissolution studies, stability analysis, and controlled substance verification
• Security & Tracking Systems -- DEA-compliant surveillance systems, biometric access control, real-time inventory tracking, and automated reporting systems
• Regulatory Compliance -- FDA registration fees, DEA licensing costs, state permits, ongoing inspection compliance, and regulatory consulting services
Economic Trends Influencing Acetaminophen/Hydrocodone Manufacturing Plant Setup Costs 2025:
• Regulatory Intensification -- Increased compliance costs due to evolving opioid regulations and enhanced monitoring requirements
• Security Technology Advancement -- Higher capital investments in sophisticated anti-diversion and tracking technologies
• Generic Market Pressure -- Pricing constraints driving operational efficiency and cost optimization initiatives
• Quality Standards Evolution -- Enhanced cGMP requirements and international regulatory harmonization increasing setup complexity
• Supply Chain Security -- Stricter raw material verification protocols and secure transportation requirements
• Abuse-Deterrent Innovation -- R&D investments in tamper-resistant formulations and delivery systems
Challenges and Considerations for Investors in Acetaminophen/Hydrocodone Manufacturing Plant Projects:
• Extreme Regulatory Complexity -- Navigating FDA, DEA, and state regulatory frameworks with lengthy approval processes and ongoing compliance obligations
• Substantial Security Investments -- Significant capital and operational expenses for physical security, personnel screening, and continuous monitoring systems
• Controlled Substance Quotas -- DEA-imposed manufacturing quotas limiting production volumes and market entry opportunities
• Market Access Barriers -- Established distribution relationships and customer contracts dominated by existing pharmaceutical companies
• Legal and Liability Risks -- Potential exposure to opioid-related litigation and regulatory enforcement actions
• Specialized Technology Requirements -- Need for advanced pharmaceutical manufacturing equipment and abuse-deterrent technologies
• Limited Skilled Workforce -- Shortage of experienced personnel with controlled substance manufacturing expertise and security clearances
Conclusion:
The acetaminophen/hydrocodone manufacturing industry in 2025 represents a highly specialized pharmaceutical sector with significant barriers to entry and extraordinary regulatory requirements. While legitimate medical demand exists for effective pain management solutions, establishing a manufacturing facility requires exceptional expertise, substantial capital investment, and unwavering commitment to regulatory compliance and security protocols.
Success in this industry demands comprehensive understanding of controlled substance regulations, significant investment in security infrastructure, and long-term dedication to maintaining the highest standards of pharmaceutical manufacturing excellence. The market is characterized by intense regulatory oversight, limited production quotas, and established competitive dynamics favoring experienced pharmaceutical companies.
Only investors with extensive pharmaceutical industry experience, substantial financial resources, and proven regulatory compliance capabilities should consider entry into acetaminophen/hydrocodone manufacturing. Companies that can demonstrate superior security protocols, regulatory expertise, and commitment to combating opioid abuse will be positioned to serve legitimate medical needs while contributing to responsible pain management therapy.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
United States: +1-631-791-1145
About Us:
IMARC Group is a global management consulting firm that helps the world's most ambitious changemakers to create a lasting impact. The company excels in understanding its client's business priorities and delivering tailored solutions that drive meaningful outcomes. We provide a comprehensive suite of market entry and expansion services. Our offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape, and benchmarking analyses, pricing and cost research, and procurement research.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Acetaminophen/Hydrocodone (Vicodin) Manufacturing Plant Report 2025 | Utility requirements and Cost Involved here
News-ID: 4158336 • Views: …
More Releases from IMARC Group
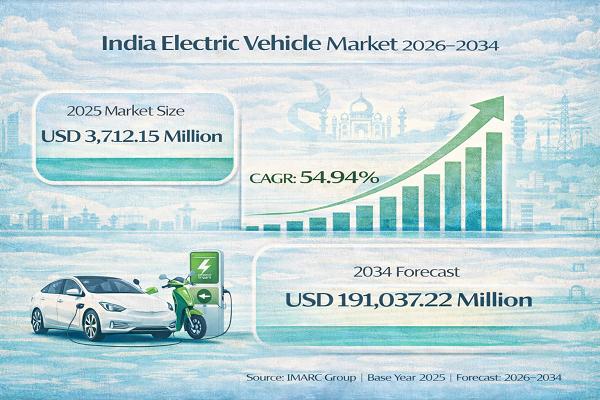
India Electric Vehicle Market Set to Reach USD 191,037.22 Million by 2034, Expan …
India Electric Vehicle Market : Report Introduction
According to IMARC Group's report titled "India Electric Vehicle Market Size, Share, Trends and Forecast by Vehicle Type, Price Category, Propulsion Type, and Region, 2026-2034" the report offers a comprehensive analysis of the industry, including market share, growth, trends, and regional insights.
Free Sample Download PDF (Exclusive Offer on Corporate Email) : https://www.imarcgroup.com/india-electric-vehicle-market/requestsample
India Electric Vehicle Market Overview
The India electric vehicle market size was valued at…
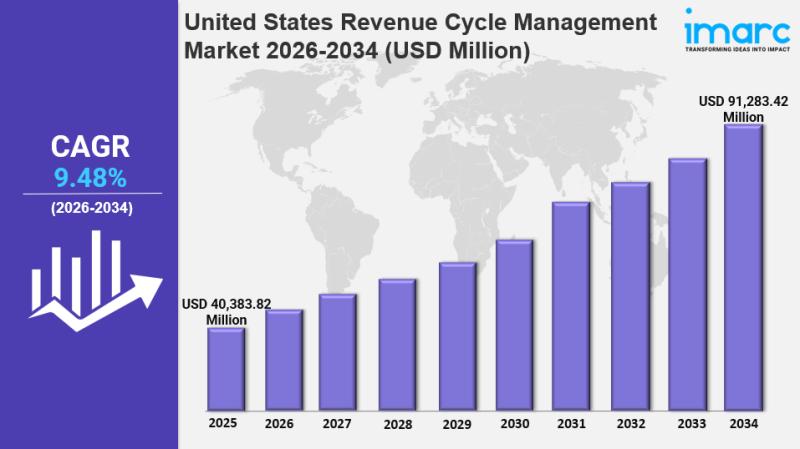
United States Revenue Cycle Management Market Size, Trends, Growth and Forecast …
IMARC Group has recently released a new research study titled "United States Revenue Cycle Management Market Size, Share, Trends and Forecast by Type, Component, Deployment, End User, and Region, 2026-2034", offers a detailed analysis of the market drivers, segmentation, growth opportunities, trends and competitive landscape to understand the current and future market scenarios.
Connect with a Research Analyst Now:
https://www.imarcgroup.com/united-states-revenue-cycle-management-market/requestsample
United States Revenue Cycle Management Market Summary:
The United States revenue cycle…

LED Chip Manufacturing Plant Cost Report 2026: Demand Analysis, CapEx/OpEx & ROI …
Setting up an LED chip manufacturing plant involves strategic planning, substantial capital investment, and comprehensive understanding of semiconductor fabrication technologies. These high-performance components power everything from general illumination and displays to automotive lighting and consumer electronics. Success requires careful site selection, advanced epitaxial growth processes, sophisticated cleanroom facilities, reliable raw material sourcing, and compliance with stringent quality and environmental regulations to ensure profitable and sustainable operations.
IMARC Group's report, "LED Chip…

Eyewear Manufacturing Plant DPR & Unit Setup - 2026: Machinery Cost, CapEx/OpEx, …
Setting up an eyewear manufacturing plant positions investors within a strategically important segment of the global optical and fashion accessories industry, driven by increasing demand for vision correction solutions, rising awareness of eye health, and growing fashion consciousness. As modern lifestyles advance, digital device usage expands, and the need for protective and corrective eyewear grows, eyewear continues to gain traction across prescription glasses, sunglasses, safety eyewear, and fashion accessories worldwide.…
More Releases for Cost
Steel Production Cost - Process Economics, Raw Materials, and Cost Drivers
Steel is the backbone of modern industry, and its production cost is one of the most closely tracked indicators across construction, infrastructure, automotive, and manufacturing sectors. Unlike niche chemicals or APIs, steel economics are driven by scale, energy intensity, and raw material volatility.
Here's the thing: steel production cost isn't just about iron ore prices. It's a layered equation involving coking coal, electricity, labor, emissions compliance, logistics, and technology choice. A…
Egg Powder Manufacturing Plant Setup Cost | Cost Involved, Machinery Cost and In …
IMARC Group's report titled "Egg Powder Manufacturing Plant Project Report 2024: Industry Trends, Plant Setup, Machinery, Raw Materials, Investment Opportunities, Cost and Revenue" provides a comprehensive guide for establishing an egg powder manufacturing plant. The report covers various aspects, ranging from a broad market overview to intricate details like unit operations, raw material and utility requirements, infrastructure necessities, machinery requirements, manpower needs, packaging and transportation requirements, and more.
In addition to…
Glucose Manufacturing Plant Cost Report 2024: Requirements and Cost Involved
IMARC Group's report titled "Glucose Manufacturing Plant Project Report 2024: Industry Trends, Plant Setup, Machinery, Raw Materials, Investment Opportunities, Cost and Revenue" provides a comprehensive guide for establishing a glucose manufacturing plant. The report covers various aspects, ranging from a broad market overview to intricate details like unit operations, raw material and utility requirements, infrastructure necessities, machinery requirements, manpower needs, packaging and transportation requirements, and more.
In addition to the operational…
Fatty Alcohol Production Cost Analysis: Plant Cost, Price Trends, Raw Materials …
Syndicated Analytics' latest report titled "Fatty Alcohol Production Cost Analysis 2023-2028: Capital Investment, Manufacturing Process, Operating Cost, Raw Materials, Industry Trends and Revenue Statistics" includes all the essential aspects that are required to understand and venture into the fatty alcohol industry. This report is based on the latest economic data, and it presents comprehensive and detailed insights regarding the primary process flow, raw material requirements, reactions involved, utility costs, operating costs, capital…
Corn Production Cost Analysis Report: Manufacturing Process, Raw Materials Requi …
The latest report titled "Corn Production Cost Report" by Procurement Resource, a global procurement research and consulting firm, provides an in-depth cost analysis of the production process of the Corn. Read More: https://www.procurementresource.com/production-cost-report-store/corn
Report Features - Details
Product Name - Corn Production
Segments Covered
Manufacturing Process: Process Flow, Material Flow, Material Balance
Raw Material and Product/s Specifications: Raw Material Consumption, Product and Co-Product Generation, Capital Investment
Land and Site Cost: Offsites/Civil Works, Equipment Cost, Auxiliary Equipment…
Crude Oil Production Cost Analysis Report: Manufacturing Process, Raw Materials …
The latest report titled "Crude Oil Production Cost Report" by Procurement Resource, a global procurement research and consulting firm, provides an in-depth cost analysis of the production process of the Crude Oil. Read More: https://www.procurementresource.com/production-cost-report-store/crude-oil
Report Features - Details
Product Name - Crude Oil
Segments Covered
Manufacturing Process: Process Flow, Material Flow, Material Balance
Raw Material and Product/s Specifications: Raw Material Consumption, Product and Co-Product Generation, Capital Investment
Land and Site Cost: Offsites/Civil Works, Equipment Cost,…
