Press release
Clinical Trial Investigative Site Network Global Market Insights: Growth Drivers, Size & Forecasts to 2029
Stay informed on tariff shifts, macro trends, and global economic changes-use code ONLINE30 to enjoy 30% off our global market reports.What Is the Forecasted Market Growth Rate of the Clinical Trial Investigative Site Network Industry?
The market size of the clinical trial investigative site network has been experiencing robust growth in the past few years. It is projected to expand from $8.01 billion in 2024 to $8.71 billion in 2025, demonstrating a compound annual growth rate (CAGR) of 8.7%. The growth witnessed in the historical period is due to several factors, including the growing instances of chronic and infectious diseases, internationalization of clinical trials, advancements in oncology, enlargement of the pharmaceutical sector, and increased governmental funding allocated towards clinical research.
How Will the Clinical Trial Investigative Site Network Market Size Evolve From 2025 to 2029?
The market size of the clinical trial investigative site network is anticipated to experience considerable growth in the coming years, expanding to $12.03 billion by 2029 with a compound annual growth rate (CAGR) of 8.4%. This projected growth in the forthcoming period can be linked to the increase in investments in adaptive and precision clinical trial designs, the surging partnerships between pharmaceutical companies and clinical site networks, the broadening of therapeutic areas beyond oncology, and the escalating number of adaptive and platform trials, as well as the expanding pipeline of biologics and personalized medicine. Key trends expected during the forecast period include the adoption of unified digital platforms, AI-enabled patient acquisition tools, the enablement of decentralized clinical trials (DCT), blockchain incorporation for ensuring data integrity and transparency, and cloud-based site management systems.
Explore The Complete Report Now:
https://www.thebusinessresearchcompany.com/report/clinical-trial-investigative-site-network-global-market-report
What Are the Main Growth Drivers in the Clinical Trial Investigative Site Network Market Today?
The surge in clinical research activity is anticipated to fuel the expansion of the clinical trial investigative site network market in the future. Clinical research activity encompasses all sustained efforts aimed at developing and interpreting clinical studies designed to advance medical knowledge or public health. The escalating demand for such activity arises from the urgent requirement for groundbreaking therapies for chronic and intricate diseases. This has amplified the pursuit of crafting safe and effective drugs and medical apparatus via thorough clinical trials. A clinical trial investigative site network bolsters clinical research activity by permitting centralized coordination, swift carrying out, and uniform quality across multiple trial sites. As an illustration, the National Library of Medicine (NLM), a US-based biomedical library, reported that there were 520,884 registered clinical trials globally in December 2024, a substantial surge from nearly 477,207 in mid-2023. This demonstrates a close to 20% growth in just over a year, underlining a sustained and vigorous escalation in global clinical research activity. Consequently, the rising demand for clinical research activity is propelling the expansion of the clinical trial investigative site network market.
Get Free Sample Report Here:
https://www.thebusinessresearchcompany.com/sample.aspx?id=25721&type=smp
Which Clinical Trial Investigative Site Network Market Segment Is Expected to Lead Through 2029?
The clinical trial investigative site network market covered in this report is segmented -
1) By Type Of Clinical Trials: Interventional Trials, Observational Trials, Expanded Access Trials, Adaptive Trials
2) By Site Type: Academic Medical Centers, Community Hospitals, Specialized Research Institutions, Private Practices, Site Management Organizations (SMOs)
3) By Application: Oncology, Cardiology, Central Nervous System, Pain Management, Endocrine, Other Applications
4) By End-Use: Pharmaceutical And Biopharmaceutical Companies, Medical Device Companies, Other End-Uses
Subsegments:
1) By Type of Interventional Trials: Phase I, Phase II, Phase III, Phase IV
2) By Type of Observational Trials: Cohort Studies, Case-Control Studies, Cross-Sectional Studies, Longitudinal Studies
3) By Type of Expanded Access Trials: Individual Patient Access, Intermediate-Size Patient Populations, Treatment IND Or Protocol Programs, Emergency Use Access
4) By Type of Adaptive Trials: Dose-Finding Adaptive Trials, Sample Size Re-estimation Trials, Adaptive Randomization Trials, Seamless Phase II Or III Trials
Which Market Trends Are Expected to Dominate Clinical Trial Investigative Site Network Industry Growth?
Leading corporations in the clinical trial investigative site network market are prioritizing the development of unique solutions such as single sign-on platforms to enhance operational efficiency, expand data accessibility, and elevate the efficiency of trials. Single sign-on platforms are authentication systems that permit users to access multiple applications or systems using a single set of login information, enhancing accessibility, security, and mitigating the need for managing numerous logins. For instance, IQVIA Inc., a firm based in the US that specializes in clinical research and health tech, in June 2024, introduced One Home for Sites. This is a unified clinical trial platform aimed at equivalizing site workflows and enhancing trial efficiency. Key features of the platform include singular sign-on access to simplify logging into numerous trial systems, central study dashboards for improved supervision of current trials, and integrated communication tools for improved interaction between sponsors, CROs, and site teams. The platform also incorporates automated workflows, document management, and real-time performance analytics that allow sites to cut down on administrative complexity, boost regulatory conformity, and expedite trial execution with an increased level of efficiency and transparency.
Customize Your Insights And Get The Full Report Here:
https://www.thebusinessresearchcompany.com/customise?id=25721&type=smp
Which Companies Hold the Largest Market Share in the Clinical Trial Investigative Site Network Sector?
Major companies operating in the clinical trial investigative site network market are IQVIA Inc., ICON plc, SGS Société Générale de Surveillance SA., Fortrea Holdings Inc., Medpace Holdings Inc., ClinChoice Inc., Velocity Clinical Research Inc., NVISION Clinical Research LLC, George Clinical, Medical Research Network Limited, MAC Clinical Research Ltd., FOMAT Medical Research Inc., Clara Health Inc., WCG Clinical, Access Clinical Trials Inc., KV Clinical Research, SMO-Pharmina, Clinical Research Network LLC, CenExel Clinical Research, EMS Healthcare Ltd.
Where Is the Clinical Trial Investigative Site Network Market Experiencing the Highest Growth?
North America was the largest region in the clinical trial investigative site network market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the clinical trial investigative site network market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa.
#Reach Out to Us#
Speak With Our Expert:
Saumya Sahay
Americas +1 310-496-7795
Asia +44 7882 955267 & +91 8897263534
Europe +44 7882 955267
Email: saumyas@tbrc.info
The Business Research Company - www.thebusinessresearchcompany.com
Follow Us On:
• LinkedIn: https://in.linkedin.com/company/the-business-research-company
Learn More About The Business Research Company
With over 15,000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Our flagship product, the Global Market Model delivers comprehensive and updated forecasts to support informed decision-making.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Clinical Trial Investigative Site Network Global Market Insights: Growth Drivers, Size & Forecasts to 2029 here
News-ID: 4156377 • Views: …
More Releases from The Business Research Company
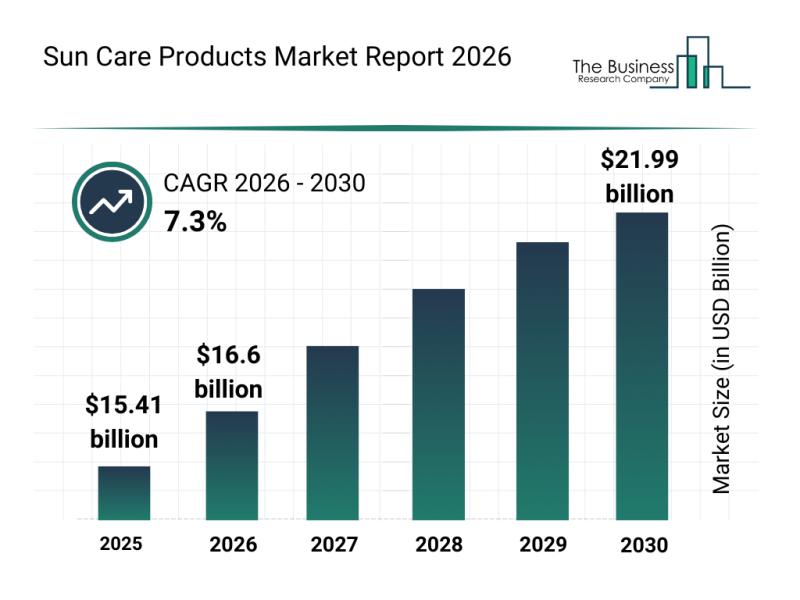
Leading Companies Fueling Growth and Innovation in the Sun Care Products Market
The sun care products market is on track for substantial expansion as consumer awareness about skin protection intensifies worldwide. With evolving preferences and technological advancements shaping product offerings, this sector is set to witness robust growth in the coming years. Let's explore the market's size projections, key players, emerging trends, and major segments driving its development through 2030.
Projected Size and Growth Trajectory of the Sun Care Products Market
The…
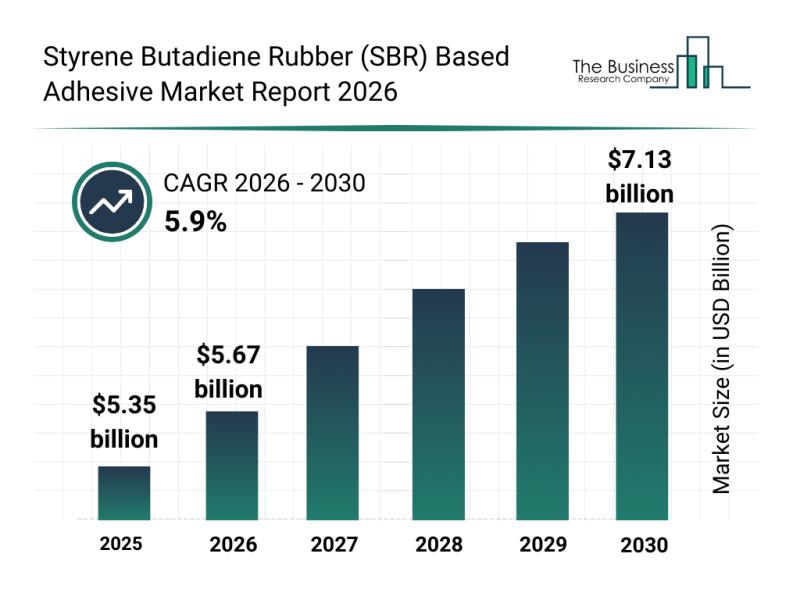
Future Perspectives: Key Trends Shaping the Styrene Butadiene Rubber (SBR) Based …
The styrene butadiene rubber (SBR) based adhesive market is on track for notable growth as we approach 2030. Driven by a variety of factors including expanding infrastructure projects and rising demand across multiple industries, this sector is poised for steady expansion. Let's explore the market's size projections, key players, emerging trends, and the main segments shaping its future.
Projected Growth and Market Size of Styrene Butadiene Rubber Based Adhesives
The…
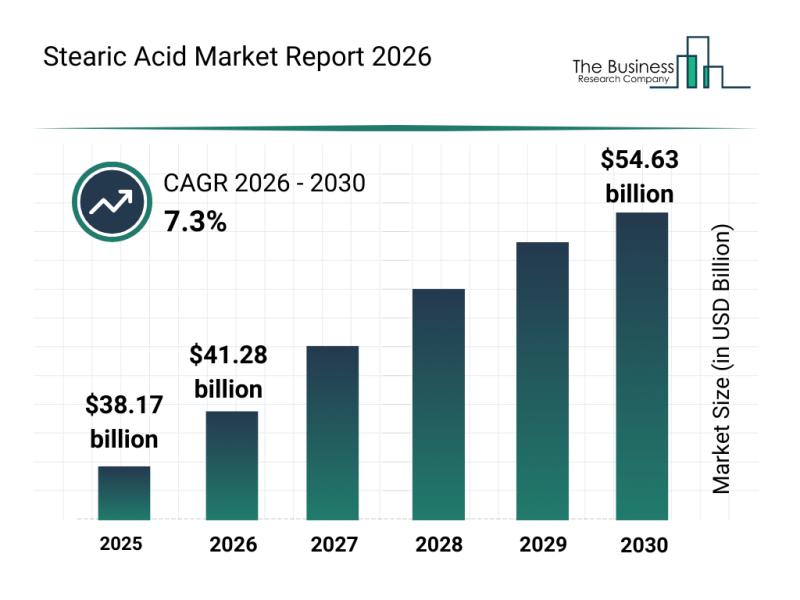
Emerging Sub-Segments Transforming the Stearic Acid Market Landscape
The stearic acid market is poised for significant expansion in the coming years, driven by evolving demand across various industries. This report explores the projected market size, leading companies, key trends, and segment analysis shaping the future of this vital chemical.
Stearic Acid Market Size and Growth Outlook
The stearic acid market is set to grow robustly, reaching a valuation of $54.63 billion by 2030. This represents a compound annual…
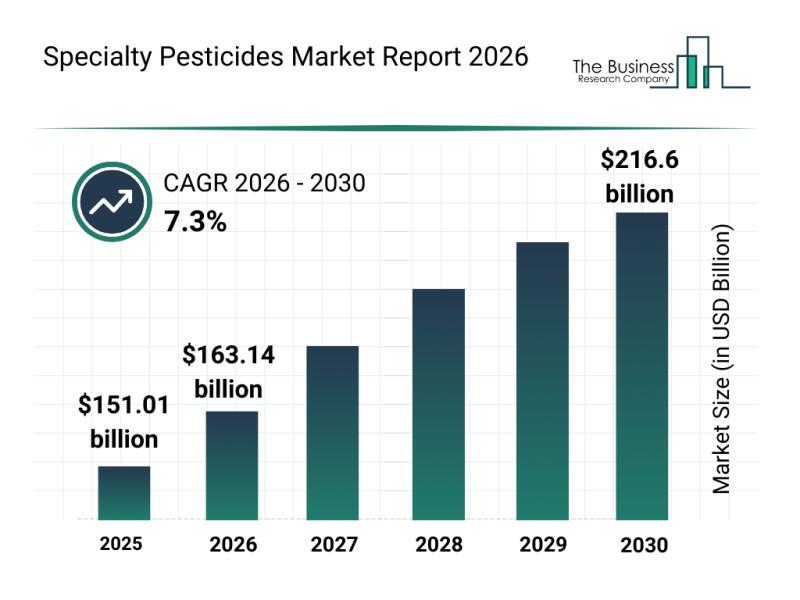
Market Trend Insights: The Impact of Recent Innovations on the Specialty Pestici …
The specialty pesticides sector is on the verge of significant expansion as global agricultural practices continue to evolve. Driven by increasing demand for crop protection and sustainable farming techniques, this market is set to experience robust growth in the coming years. Let's explore the market's anticipated value, leading companies, emerging trends, and detailed segmentation to gain a comprehensive understanding of this dynamic industry.
Projected Market Size and Growth Expectations for Specialty…
More Releases for Trial
Clinical Trial Investigative Site Network Market Clinical Trial Investigative Si …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trial Investigative Site Network Market - (By Therapeutic Areas (Oncology, Cardiology, CNS, Pain Management, Endocrine, Others), By Phase (Phase I, Phase II, Phase III, Phase IV), By End-use (Sponsor, CRO)), Trends, Industry Competition Analysis, Revenue and Forecast To 2034."
According to the latest research by InsightAce Analytic, the Global Clinical Trial Investigative Site Network Market…
Transformative Trends Impacting the Electronic Trial Master File (eTMF) Systems …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Electronic Trial Master File (eTMF) Systems Market Size By 2025?
The market size of the electronic trial master file (eTMF) systems has experienced fast growth over recent years. The market is projected to increase from $1.36 billion in 2024 to $1.55 billion in 2025, with…
Transformative Trends Impacting the Electronic Trial Master File (eTMF) Systems …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Electronic Trial Master File (eTMF) Systems Market Size By 2025?
The market size of the electronic trial master file (eTMF) systems has experienced fast growth over recent years. The market is projected to increase from $1.36 billion in 2024 to $1.55 billion in 2025, with…
Clinical Trial Imaging market
The Clinical Trial Imaging market crossed the US$ 1.09 billion mark in 2022 and is expected to hit US$ 1.94 billion by 2030, recording a CAGR of 7.5% during the forecast period.
Rising R&D spending, a rapidly growing pharmaceutical industry, and an increase in the number of contract research organizations are some of the major factors driving the market's growth. There has been an increase in pharmaceutical companies due to the…
Clinical Trial Logistics
Clinical Trial Logistics
16th to 17th May 2011, Marriott Regents Park, London, United Kingdom.
It currently costs just over £500 million ($800 million) to bring a new chemical to market and development timelines continue to fall in the 10-15 year range. A key reason for high R&D costs is due to logistical failures including failure to recruit patients on time. A way to avoid this is to move clinical trials…
Clinical Trial Logistics
Announcing SMi's 5th annual…
Clinical Trial Logistics conference
16th and 17th May 2011, Central London, UK
www.smi-online.co.uk/2011logistics-london6.asp
It currently costs just over £500 million ($800 million) to bring a new chemical to market and development timelines continue to fall in the 10-15 year range. A key reason for high R&D costs is due to logistical failures including failure to recruit patients on time. A way to avoid this is to move clinical…
