Press release
Orthopedic Trauma Devices Market Poised to Register a CAGR of ~6.43% by 2032 | DelveInsight
The orthopedic trauma devices market is experiencing robust growth, driven by the rising incidence of road traffic accidents, sports-related injuries, and age-related orthopedic conditions such as osteoporosis and fractures. With the global population aging rapidly and urbanization contributing to higher accident rates, the demand for advanced trauma fixation products, including plates, screws, intramedullary nails, and external fixators, is expanding significantly.Technological advancements, such as bioabsorbable implants, 3D-printed devices, and minimally invasive surgical techniques, are reshaping treatment outcomes, offering patients faster recovery and improved mobility. Furthermore, the integration of smart implants with sensors and real-time monitoring capabilities is emerging as a transformative trend, enhancing post-surgical care and rehabilitation.
As healthcare systems worldwide emphasize value-based care and efficient fracture management, adoption of innovative orthopedic trauma solutions is expected to accelerate. Consequently, the market is projected to maintain steady growth momentum through 2032, supported by investments in R&D and the increasing availability of trauma care facilities across both developed and emerging economies.
DelveInsight's Orthopedic Trauma Devices Market Insights report [https://www.delveinsight.com/report-store/orthopedic-trauma-devices-market?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=jpr] provides a detailed evaluation of current and forecast market dynamics, key growth drivers, barriers, regulatory landscape, competitive positioning, and in-depth profiles of leading companies shaping the future of the orthopedic trauma devices market.
Key Takeaways from the Orthopedic Trauma Devices Market Report
- The orthopedic trauma devices market was valued at USD 7.01 billion in 2023 and is projected to reach USD 10.15 billion by 2032, growing at a CAGR of 6.43% from 2025 to 2032.
- As per DelveInsight estimates, North America is anticipated to dominate the global Orthopedic Trauma Devices market during the forecast period.
- Notable Orthopedic Trauma Devices companies such as Zimmer Biomet, Orthofix Medical Inc., Johnson & Johnson Services, Inc., B. Braun Meslungen AG, Stryker, Medtronic, Acumed, INION OY, Orthomed, Smith & Nephew Plc, CONMED Corporation, Bioretec Ltd., Arthrex Inc., JEIL MEDICAL CORPORATION, OsteoMed, Medartis AG, Biorez, Invibio Ltd., Advanced Orthopaedic Solutions, DJO, LLC, and several others are currently operating in the Orthopedic Trauma Devices market.
- In May 2025, Massachusetts-based Trax Surgical, LLC received FDA 510(k) clearance for its LINKT Trademark Compression Staple System, designed for fracture repair, joint fusion, and osteotomy procedures in orthopedic extremities.
- In April 2025, Ventris Medical announced U.S. FDA 510(k) clearance for Backpack Registered (Porous Biologic Scaffold) for intervertebral disc use. Designed to promote cell growth and bone formation, Backpack Registered supports orthopedic and spinal fusion surgeries and is available with either osteoinductive Allocell Registered AF fibers or surface-activated Amplify Registered granules.
- In February 2025, Atreon Orthopedics, LLC, a Columbus-based innovator in tissue healing, announced FDA 510(k) clearance and the full market launch of BioCharge Registered Autobiologic Matrix. This bioresorbable synthetic implant is designed to improve rotator cuff repair integrity and long-term patient outcomes.
- In October 2024, Globus Medical, Inc., a leading musculoskeletal solutions company, announced the continued growth and expansion of its orthopedic trauma product portfolio. Globus introduced several new system extensions in 2024 and received 510(k) clearance from the U.S. Food and Drug Administration for its first suture-based product, the TENSOR Trademark Suture Button System. Among the newly launched next-generation systems were the ANTHEM Trademark II Distal Radius Volar Plates, AUTOBAHN Trademark Trochanteric Nail PRO Instruments, and CAPTIVATE Trademark SOLA Headless Screws.
To read more about the latest highlights related to the Orthopedic Trauma Devices market, get a snapshot of the key highlights entailed in the Global Orthopedic Trauma Devices Market Report [https://www.delveinsight.com/report-store/orthopedic-trauma-devices-market?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=jpr]
Orthopedic Trauma Devices Overview
Orthopedic trauma devices are medical implants and tools designed to treat injuries to the bones and soft tissues, such as fractures, dislocations, and ligament tears. These devices-including screws, plates, rods, pins, and external fixators-are used to stabilize and support the affected area, ensuring proper alignment and faster healing. They play a critical role in trauma care, particularly following accidents, falls, or sports injuries. With the growing global incidence of orthopedic injuries, aging populations, and a shift toward minimally invasive surgical techniques, the orthopedic trauma devices market is witnessing steady growth, driven by continuous innovation and improved surgical outcomes.
Orthopedic Trauma Devices Market Insights
North America is expected to dominate the orthopedic trauma devices market from 2023 through 2032, driven by a high incidence of hip fractures, avascular necrosis, and shoulder injuries, along with a strong healthcare infrastructure and presence of major players like Johnson & Johnson, Stryker, and Zimmer Biomet. According to the American Academy of Orthopaedic Surgeons (2024), over 300,000 hip fractures occur annually in the U.S., primarily in individuals aged 65 and older. The NIH (2023) reports 20,000-30,000 new avascular necrosis cases each year, while data from the American Academy of Physical Medicine and Rehabilitation (2022) highlights a high burden of shoulder dislocations, proximal humerus fractures, and clavicle injuries. Orthopedic trauma devices such as internal fixators, prosthetics, plates, and external supports play a critical role in managing these injuries, aiding in pain relief, bone stabilization, and functional recovery. With advanced medical services and strong distribution networks ensuring accessibility, the region remains a key driver of market growth through the forecast period.
To know more about why North America is leading the market growth in the Orthopedic Trauma Devices market, get a snapshot of the Orthopedic Trauma Devices Market Outlook [https://www.delveinsight.com/report-store/orthopedic-trauma-devices-market?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=jpr]
Orthopedic Trauma Devices Market Dynamics
According to the World Health Organization (2023), around 344 million people globally are affected by osteoarthritis, with 73% over the age of 55 and 60% being female. Additionally, 13 million individuals are living with rheumatoid arthritis. These degenerative joint conditions contribute significantly to the demand for orthopedic trauma devices, which help manage joint damage, inflammation, and loss of mobility. Sports and recreational injuries also drive this demand, data from the National Safety Council (2024) reported 3.7 million emergency room visits in 2023 for such injuries, with a notable 8% rise in exercise-related injuries. Furthermore, the global burden of hip fractures is rising, especially in Asia, where over 50% of osteoporotic hip fractures are projected to occur by 2050.
These growing orthopedic needs have underscored the importance of trauma devices in joint stabilization, mobility restoration, and fracture recovery. However, despite their benefits, risks such as nerve or blood vessel injury, stiffness, and complications from device placement may limit their use, posing challenges to market expansion.
Get a sneak peek at the Orthopedic Trauma Devices market dynamics @ https://www.delveinsight.com/report-store/orthopedic-trauma-devices-market [https://www.delveinsight.com/report-store/orthopedic-trauma-devices-market?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=jpr]
Coverage: Global
Study Period: 2022 to 2032
Orthopedic Trauma Devices Market CAGR: ~6.43%
Key Orthopedic Trauma Devices Companies: Zimmer Biomet, Orthofix Medical Inc., Johnson & Johnson Services, Inc., B. Braun Meslungen AG, Stryker, Medtronic, Acumed, INION OY, Orthomed, Smith & Nephew Plc, CONMED Corporation, Bioretec Ltd., Arthrex Inc., JEIL MEDICAL CORPORATION, OsteoMed, Medartis AG, Biorez, Invibio Ltd., Advanced Orthopaedic Solutions, DJO, LLC, and others.
Orthopedic Trauma Devices Market Segmentation
Market Segmentation By Product Type: Internal Fixators [Plates & Screws, Rods & Pins, and Others] and External Fixators [Unilateral, Circular, Hybrid, and others].
Market Segmentation By Absorbability: Non-Absorbable and Resorbable
Market Segmentation By End User: Hospitals, Ambulatory Surgical Centers, and others.
Market Segmentation By Geography: North America, Europe, Asia-Pacific, and Rest of the World.
Which MedTech key players in the Orthopedic Trauma Devices market are set to emerge as the trendsetter, explore @ Orthopedic Trauma Devices Companies [https://www.delveinsight.com/report-store/orthopedic-trauma-devices-market?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=jpr]
Table of Contents
1. Electrophysiology Devices Market Report Introduction
2. Electrophysiology Devices Market Executive Summary
3. Competitive Landscape
4. Regulatory Analysis
5. Electrophysiology Devices Market Key Factors Analysis
6. Electrophysiology Devices Market Porter's Five Forces Analysis
7. Electrophysiology Devices Market Layout
8. Electrophysiology Devices Market Company and Product Profiles
9. KOL Views
10. Project Approach
11. About DelveInsight
12. Disclaimer & Contact Us
About DelveInsight
DelveInsight is a premier healthcare business consultant and market research firm, specializing in life sciences. We empower pharmaceutical companies with comprehensive end-to-end solutions designed to enhance performance and drive growth.
Our expert healthcare consulting services offer in-depth market analysis, helping businesses accelerate growth and navigate challenges with actionable, results-driven strategies.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Jatin Vimal
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=orthopedic-trauma-devices-market-poised-to-register-a-cagr-of-643-by-2032-delveinsight]
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: Nevada
Country: United States
Website: https://www.delveinsight.com/
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Orthopedic Trauma Devices Market Poised to Register a CAGR of ~6.43% by 2032 | DelveInsight here
News-ID: 4155035 • Views: …
More Releases from ABNewswire

Cataract Surgery Devices Market to Grow at a CAGR of ~5.50% by 2032, Driven by A …
The cataract surgery devices market is steadily growing due to the rising prevalence of cataracts, the leading cause of global blindness, and increased use of advanced surgical techniques. According to WHO, cataracts cause nearly 51% of blindness worldwide, mainly affecting aging populations. Longer life expectancy and improved healthcare in emerging markets are driving demand for effective and affordable cataract surgery solutions.
Technological innovations such as femtosecond laser-assisted cataract surgery (FLACS), premium…

Diabetic Nephropathy Pipeline Outlook 2025: Clinical Trial Studies, EMA, PDMA, F …
DelveInsight's, "Diabetic Nephropathy Pipeline Insight, 2025" report provides comprehensive insights about 20+ companies and 25+ pipeline drugs in Diabetic Nephropathy pipeline landscape. It covers the Diabetic Nephropathy pipeline drug profiles, including clinical and nonclinical stage products. It also covers the Diabetic Nephropathy therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive Diabetic Nephropathy pipeline products in this space.
Explore the comprehensive insights by…
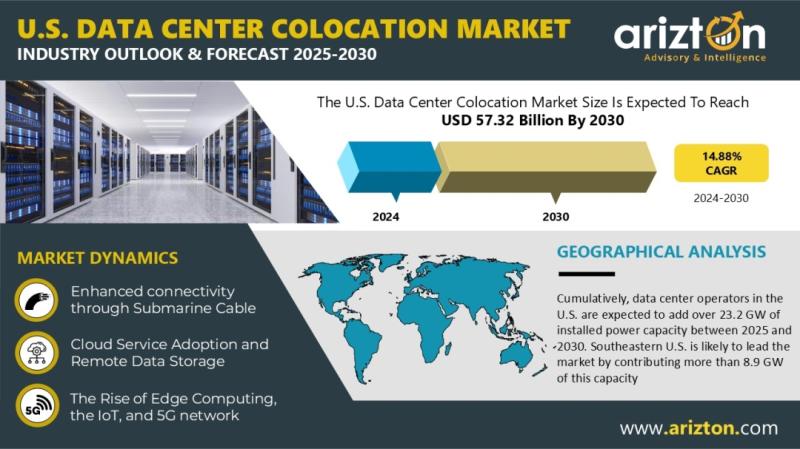
U.S. Data Center Colocation Market Investment to Reach USD 57.32 Billion by 2030 …
Industry Analysis Report, Regional Outlook, Growth Potential, Price Trends, Competitive Market Share & Forecast 2025-2030.
According to Arizton latest research report, U.S. data center colocation market [https://www.arizton.com/market-reports/us-data-center-colocation-market] is growing at a CAGR of 14.88% during 2024-2030.
Looking for More Information? Click: [https://www.arizton.com/market-reports/us-data-center-colocation-market]
Report Scope:
Market Size - Investment (2030): USD 57.32 Billion
Market Size - Investment (2024): USD 24.94 Billion
CAGR - Investment (2024-2030):14.88%
Market Size - Area (2030):18.20 Million Square Feet
Market Size - Power Capacity (2030):4,520…

Rising Trend of Self-Reliant Renovations Fuels Global DIY Home Improvement Marke …
DIY Home Improvement Market is anticipated to reach USD 1173990 million by 2032 with a Compound Annual Growth Rate (CAGR) of 4.5 % from 2026 to 2032. Primary market drivers include Rising Renovation Demand, Growing Homeownership, Technological advancement and Digitalization.
The latest premium report by Profshare Market Research, " DIY Home Improvement Market [https://www.profsharemarketresearch.com/diy-home-improvement-market/] by Product Type (Building Materials, Paints and Wallpaper, Decor and Indoor Garden, Plumbing Materials and Equipment, Tools…
More Releases for Devices
Spinal Fusion Devices Market Size to Reach Valuation of $7.43 Billion by 2022 | …
Increase in adoption of minimally invasive spine surgery (MISS) presents lucrative opportunities for key players in the spinal fusion devices market. MISS is preferred to conventional techniques, owing to its associated benefits such as minimal cut or incision, which in turn reduces the chances of damage caused to the adjacent muscles.
Spinal fusion devices market was valued at $5,867 million in 2015, and is projected to reach $7,435 million by 2022,…
Global Beauty Devices Market Industry Insights Forecast to 2024, Coverage Cellul …
The global beauty devices market was valued at USD 39.1 billion in 2018 and is anticipated to grow at a CAGR of 18.4% during the forecast period. The significant growth in the beauty devices industry is imputed to the rise in prevalence of skin disorders, increasing rate of hormonal imbalance cases, an increase in the geriatric population, and growing awareness for beauty devices.
Request for Free Sample Copy of this Research…
Canada Anesthesia and Respiratory Devices Market Segments Including Airway Manag …
ReportsnReports added a new report on The Canada Anesthesia and Respiratory Devices Market report that delivers the clean elaborated structure of the Report comprising each and every business-related information of the market at a global level. The in-depth study on the current state which focuses on the major drivers and restraints for the key players. Canada Anesthesia and Respiratory Devices Market Industry research report provides granular analysis of the market…
Global Beauty Devices Market Insights 2018 By Products Hair Growth Devices,Acne …
Description
This report studies the global market size of Beauty Devices in key regions like North America, Europe, Asia Pacific, Central & South America and Middle East & Africa, focuses on the consumption of Beauty Devices in these regions.
This research report categorizes the global Beauty Devices market by players/brands, region, type and application. This report also studies the global market status, competition landscape, market share, growth rate, future trends, market drivers,…
Wearable Electronic Devices Market,Wearable Electronic Devices Industry, Global …
Latest industry research report on: Global Wearable Electronic Devices Market : Industry Size, Share, Research, Reviews, Analysis, Strategies, Demand, Growth, Segmentation, Parameters, Forecasts
This report studies the global Wearable Electronic Devices market status and forecast, categorizes the global Wearable Electronic Devices market size (value & volume) by manufacturers, type, application, and region. This report focuses on the top manufacturers in United States, Europe, China, Japan, South Korea and Taiwan and other…
Ophthalmic Devices Market By Product Function [Ophthalmic Surgery Devices (Refra …
Ophthalmology is a branch of medical sciences that deals with the structure, function, and various eye diseases. The ophthalmic devices are medical equipment designed for diagnosis, surgical, and vision correction purposes. These devices gain increased importance and adoption due to high prevalence of various ophthalmic diseases such as glaucoma, cataract, and other vision related issues.
Request Sample At: https://www.bigmarketresearch.com/request-sample/1074435
Increase in prevalence rate of eye related diseases such as glaucoma, cataract,…
