Press release
US Biosimilars Market is expected to reach US$ 65.62 billion by 2033 | DataM Intelligence
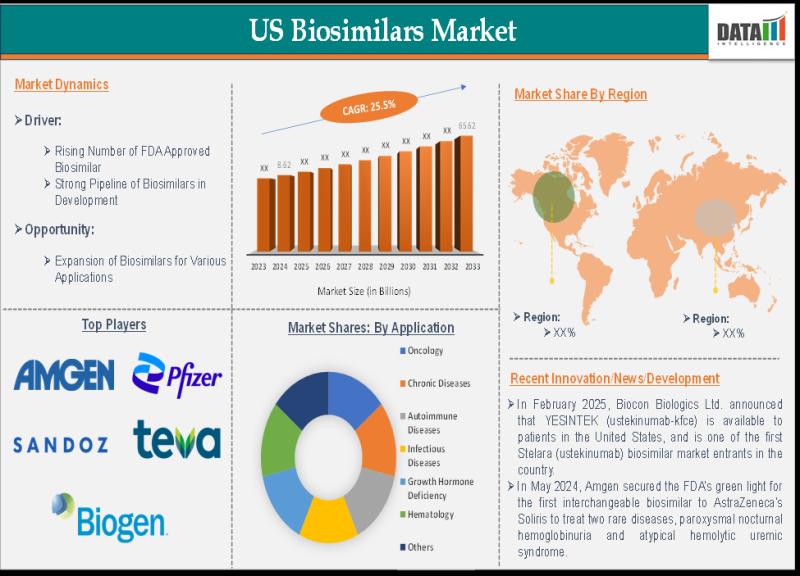
Overview of the Market:The U.S. Biosimilars Market was valued at approximately USD 8.62 billion in 2024 and is projected to reach around USD 65.62 billion by 2033, growing at a compound annual growth rate (CAGR) of 25.5% from 2025 to 2033 . This robust growth trajectory is driven by several factors, including the expiration of patents for blockbuster biologics, increasing healthcare costs, and the need for more affordable treatment options.
The oncology segment leads the market, primarily due to the high prevalence of cancer and the substantial cost burden associated with its treatment. Monoclonal antibodies, such as those targeting tumor necrosis factor (TNF) and interleukin pathways, dominate the product type segment, owing to their widespread use and significant cost savings compared to their reference products .
Get a Sample PDF Brochure of the Report (Use Corporate Email ID for a Quick Response): https://www.datamintelligence.com/download-sample/us-biosimilars-market?sz
Key Highlights from the Report:
➤ The U.S. biosimilars market is projected to grow at a CAGR of 25.5% from 2025 to 2033.
➤ Oncology is the leading application segment, driven by the high cost of cancer treatments.
➤ Monoclonal antibodies represent the dominant product type in the biosimilars market.
➤ The increasing number of patent expirations for biologics is a significant growth driver.
➤ Rising healthcare costs and demand for affordable therapies are fueling market expansion.
➤ Strategic partnerships and collaborations are enhancing biosimilar development and market penetration.
Market Segmentation:
The U.S. biosimilars market can be segmented based on product type, application, and distribution channel:
Product Type
Monoclonal Antibodies: These are the most widely used biosimilars, targeting diseases like rheumatoid arthritis, Crohn's disease, and various cancers.
Recombinant Human Growth Hormone (rhGH): Used in growth hormone deficiencies, this segment is witnessing increased adoption due to cost-effectiveness.
Insulin: Biosimilar insulins are gaining traction in diabetes management, offering more affordable options for patients.
Other Products: Includes erythropoietin, granulocyte colony-stimulating factor, and interferons, each serving specific therapeutic areas.
Application
Oncology: The largest application segment, driven by the high incidence of cancer and the cost of biologic therapies.
Chronic Diseases: Includes conditions like rheumatoid arthritis and diabetes, where biosimilars offer significant cost savings.
Autoimmune Diseases: Biosimilars are increasingly used in treating autoimmune disorders, providing effective alternatives to original biologics.
Others: Encompasses various therapeutic areas benefiting from biosimilar treatments.
Distribution Channel
Hospital Pharmacies: The primary channel for biosimilars, given the centralized nature of hospital drug procurement.
Retail Pharmacies: Increasingly dispensing biosimilars as patient demand for affordable options rises.
Specialty Pharmacies: Focused on complex biologic therapies, including biosimilars, catering to specialized patient needs.
Looking For Full Report? Get it Here: https://www.datamintelligence.com/buy-now-page?report=us-biosimilars-market
Regional Insights:
The U.S. biosimilars market exhibits regional variations influenced by healthcare infrastructure, patient demographics, and local regulations:
Northeast: Home to leading healthcare institutions and a high adoption rate of biosimilars.
Midwest: Growing acceptance due to cost-saving initiatives by healthcare providers.
South: Increasing awareness and adoption, driven by state-level healthcare reforms.
West: Significant market due to a large patient population and progressive healthcare policies.
Market Dynamics:
Market Drivers
Patent Expirations: The impending loss of exclusivity for several blockbuster biologics is opening opportunities for biosimilar development and market entry.
Cost Savings: Biosimilars offer substantial cost reductions, making therapies more accessible to patients and healthcare systems.
Regulatory Support: The FDA's streamlined approval processes for biosimilars are accelerating their availability in the market.
Market Restraints
Regulatory Challenges: Complex approval pathways and interchangeability requirements can delay biosimilar market entry.
Market Acceptance: Physicians' and patients' reluctance to switch from established biologics to biosimilars can hinder adoption.
Reimbursement Issues: Inconsistent insurance coverage and reimbursement policies can affect biosimilar uptake.
Market Opportunities
Expanding Therapeutic Areas: Biosimilars are being developed for new indications, broadening their market potential.
International Expansion: U.S. biosimilar manufacturers have opportunities to enter emerging markets with growing healthcare needs.
Innovative Formulations: Development of biosimilars with improved delivery mechanisms or formulations can enhance patient compliance and market share.
Speak to Our Analyst and Get Customization in the report as per your requirements: https://www.datamintelligence.com/customize/us-biosimilars-market
Reasons to Buy the Report:
✔ Comprehensive analysis of the U.S. biosimilars market, including current trends and future forecasts.
✔ In-depth segmentation by product type, application, and distribution channel.
✔ Insights into regional market dynamics and adoption rates.
✔ Detailed examination of market drivers, restraints, and opportunities.
✔ Strategic recommendations for stakeholders to navigate the evolving biosimilars landscape.
Frequently Asked Questions (FAQs):
◆ How Big is the U.S. Biosimilars Market?
◆ Who are the Key Players in the U.S. Biosimilars Market?
◆ What is the Projected Growth Rate of the U.S. Biosimilars Market?
◆ What is the Market Forecast for 2034?
◆ Which Region is Estimated to Dominate the U.S. Biosimilars Market?
Company Insights:
Key players in the U.S. biosimilars market include:
• Amgen Inc.: A pioneer in biosimilars, with a robust portfolio and ongoing development programs.
• Sandoz (Novartis): A leader in biosimilar manufacturing and commercialization.
• Pfizer Inc.: Expanding its biosimilar offerings across various therapeutic areas.
• Samsung Bioepis: Collaborating with global partners to bring biosimilars to market.
• Biocon Biologics: A significant player focusing on oncology and autoimmune biosimilars.
Recent developments:
Amgen: Launched a new biosimilar targeting a leading oncology drug, expanding its market presence.
Sandoz: Entered a strategic partnership with a U.S.-based healthcare provider to enhance biosimilar adoption.
Conclusion:
The U.S. Biosimilars Market is poised for significant growth, driven by patent expirations, cost-saving opportunities, and supportive regulatory frameworks. As the healthcare landscape continues to evolve, biosimilars will play a crucial role in providing affordable and effective treatment options for patients across the country. Stakeholders, including healthcare providers, payers, and pharmaceutical companies, must navigate this dynamic market to capitalize on emerging opportunities and address the challenges ahead.
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release US Biosimilars Market is expected to reach US$ 65.62 billion by 2033 | DataM Intelligence here
News-ID: 4148101 • Views: …
More Releases from DataM Intelligence 4Market Research
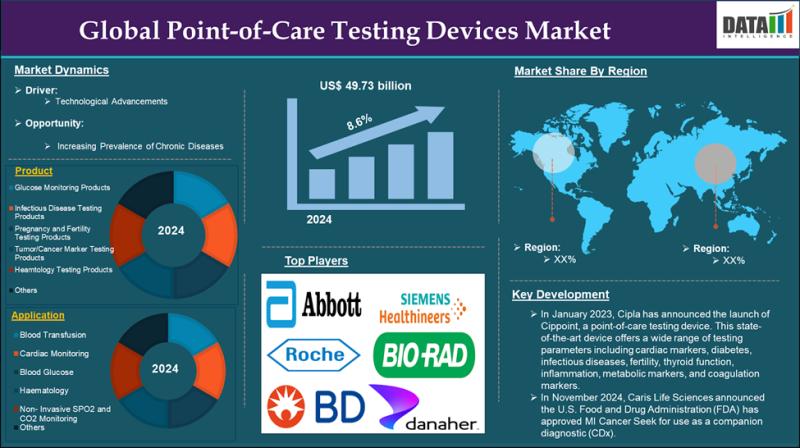
Point-of-Care Testing Devices Market is expected to reach US$ 101.51 billion by …
Market Size and Growth:
The Global Point-of-Care Testing Devices Market size reached US$ 49.73 billion in 2024 and is expected to reach US$ 101.51 billion by 2033, growing at a CAGR of 8.6% during the forecast period 2025-2033.
The Point-of-Care Testing Devices Market encompasses diagnostic tools and technologies that enable rapid, on-site medical testing near the patient, eliminating the need for centralized laboratories. These devices provide immediate results for various conditions, including…
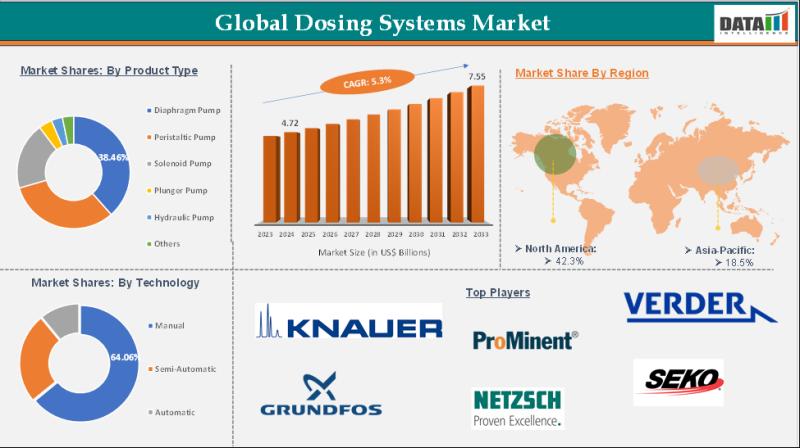
Dosing Systems Market is expected to reach US$ 7.55 Billion by 2033 | Major key …
Market Size and Growth:
The Dosing Systems Market size reached US$ 4.72 Billion in 2024 and is expected to reach US$ 7.55 Billion by 2033, growing at a CAGR of 5.3% during the forecast period 2025-2033.
The Dosing Systems Market encompasses the global industry involved in the development, manufacturing, and supply of precise fluid or chemical dosing equipment used across various sectors, including water treatment, chemicals, food & beverages, pharmaceuticals, and agriculture.…
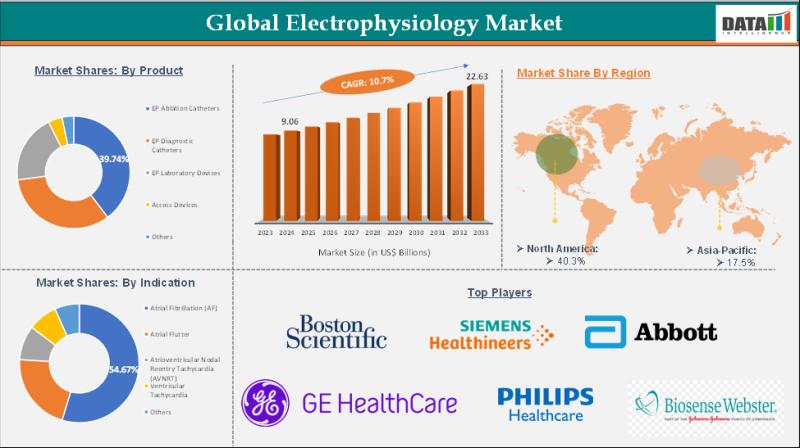
Electrophysiology Market is expected to reach US$ 22.63 Billion by 2033 | Major …
Market Size and Growth:
The Electrophysiology Market reached US$ 9.06 Billion in 2024 and is expected to reach US$ 22.63 Billion by 2033, growing at a CAGR of 10.7% during the forecast period 2025-2033.
The Electrophysiology Market encompasses the global industry focused on the diagnosis, monitoring, and treatment of heart rhythm disorders through advanced electrophysiology (EP) procedures, devices, and technologies. It includes EP catheters, mapping systems, ablation equipment, and implantable cardiac devices…
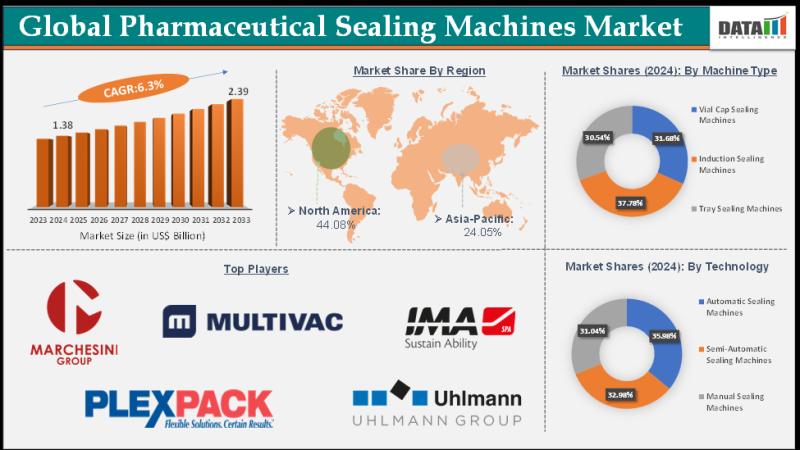
Pharmaceutical Sealing Machines Market is expected to reach US$ 2.39 Billion by …
Market Size and Growth:
The Pharmaceutical Sealing Machines Market size reached US$ 1.38 Billion in 2024 and is expected to reach US$ 2.39 Billion by 2033, growing at a CAGR of 6.3% during the forecast period 2025-2033.
The Pharmaceutical Sealing Machines Market encompasses the global industry involved in the manufacturing, distribution, and sale of machines designed to seal pharmaceutical products such as tablets, capsules, vials, bottles, and blister packs. These machines ensure…
More Releases for Biosimilar
Interchangeable Biosimilar Humira Market Share Driven by Biologic Therapy Adopti …
Interchangeable Biosimilar Humira Market
The global market for Interchangeable Biosimilar Humira was valued at US$ million in the year 2024 and is projected to reach a revised size of US$ million by 2031, growing at a CAGR of %during the forecast period
View sample report
https://reports.valuates.com/request/sample/QYRE-Auto-33I15005/Global_Interchangeable_Biosimilar_Humira_Market_Research_Report_2023
The Interchangeable Biosimilar Humira Market is experiencing significant market growth as healthcare providers and patients increasingly adopt biosimilar therapies for autoimmune and inflammatory conditions. Market trends indicate rising…
Key Trend Reshaping the Biosimilar Monoclonal Antibodies Market in 2025: Advance …
What Are the Projections for the Size and Growth Rate of the Biosimilar Monoclonal Antibodies Market?
In recent times, the biosimilar monoclonal antibodies sector has experienced a swift expansion. The market size, which stands at $8.04 billion in 2024, is projected to climb to $9.25 billion in 2025, marking a compound annual growth rate (CAGR) of 15.1%. Factors such as expired patents, an increased understanding of biosimilars, governmental strategies, heightened financial…
Key Trend Reshaping the Biosimilar Monoclonal Antibodies Market in 2025: Advance …
What Are the Projections for the Size and Growth Rate of the Biosimilar Monoclonal Antibodies Market?
In recent times, the biosimilar monoclonal antibodies sector has experienced a swift expansion. The market size, which stands at $8.04 billion in 2024, is projected to climb to $9.25 billion in 2025, marking a compound annual growth rate (CAGR) of 15.1%. Factors such as expired patents, an increased understanding of biosimilars, governmental strategies, heightened financial…
Biosimilar Market Treating More for Less: The Booming Infliximab Biosimilar Mark …
Infliximab Biosimilar Market worth $ XX Million by 2030 - Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Infliximab Biosimilar Market- by Application (Crohn's Disease, Psoriatic Arthritis, Rheumatoid Arthritis, Ulcerative Colitis, Ankylosing Spondylitis, Plaque Psoriasis and Others), End User (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy and Other Direct Distribution Channels), Trends, Industry Competition Analysis, Revenue and Forecast To 2030."
Get…
Biosimilar Monoclonal Antibodies Market
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the " "Global Biosimilar Monoclonal Antibodies Market by Product (infliximab, trastuzumab, rituximab, adalimumab, bevacizumab, cetuximab, ranibizumab, denosumab, eculizumab, and other pipeline products), Indication (oncology, inflammatory & autoimmune disorders, chronic diseases, blood disorders, and other indications), Clinical Trial/Pipeline Analysis, Future Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
The Biosimilar Monoclonal Antibodies Market Size is valued at 5.02…
Infliximab Biosimilar Insight, 2022 | DelveInsight
DelveInsight's, "Infliximab Biosimilar Insight, 2022" report provides comprehensive insights about 35+ companies and 45+ marketed and pipeline drugs in Infliximab Biosimilars landscape. It covers the marketed and pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Interested to know more about the functioning of…