Press release
Vertigo Pipeline Insight 2025: Novel Drug Classes and Central Mechanism Targets Drive Innovation | DelveInsight
The vertigo treatment pipeline is steadily advancing, with numerous companies exploring novel therapies targeting its complex causes. Common in older adults, vertigo is often managed with vestibular suppressants or antiemetics, which provide only short-term relief. Growing demand for targeted, disease-modifying treatments is driving continued R&D in both central and peripheral vestibular pathway modulation.DelveInsight's "Vertigo - Pipeline Insight, 2025 [https://www.delveinsight.com/report-store/vertigo-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=jpr]" highlights the growing momentum in drug development focused on vestibular disorders, particularly in conditions such as Meniere's disease, vestibular migraine, and bilateral vestibulopathy. Companies are leveraging advances in neuropharmacology and inner ear biology to explore diverse therapeutic strategies. Drug candidates such as betahistine analogs, neurokinin receptor antagonists, selective serotonin receptor modulators, and sodium channel blockers are showing promise in early to mid-stage clinical trials. Several pipeline assets are designed to act on central nervous system targets involved in vestibular compensation and sensory mismatch processing.
Among the most advanced candidates is SENS-401 (Aeglea BioTherapeutics), initially developed for sudden sensorineural hearing loss but also evaluated for vestibular balance disorders. Another notable candidate is SPI-1005 (ebselen), developed by Sound Pharmaceuticals, which modulates oxidative stress and inflammation in the inner ear. Efforts are also underway to evaluate non-invasive vestibular rehabilitation technologies in combination with pharmacologic agents, with an aim to enhance neural plasticity and functional recovery in chronic vertigo patients.
As the understanding of vestibular dysfunction deepens, the 2025 pipeline reflects a strategic shift from generic symptomatic agents toward mechanism-specific drugs with neuroprotective or restorative potential. Regulatory designations such as Fast Track and orphan drug status are helping accelerate development in niche subtypes like Meniere's disease. With a blend of CNS-focused candidates and inner ear-targeted therapies in the pipeline, the coming years may see a meaningful transformation in the clinical management of vertigo and related disorders.
Interested in learning more about the current treatment landscape and the key drivers shaping the Vertigo pipeline? Click here [https://www.delveinsight.com/report-store/vertigo-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=jpr]
Key Takeaways from the Vertigo Pipeline Report
- DelveInsight's Vertigo pipeline analysis depicts a strong space with 2+ active players working to develop 2+ pipeline drugs for Vertigo treatment.
- The leading Vertigo companies include Auris Medical, Sensorion, Apsen Farmaceutica, and others are evaluating their lead assets to improve the Vertigo treatment landscape.
- Key Vertigo pipeline therapies in various stages of development include AM-125, Seliforant, APSLXR, and others.
- In March 2025, A pilot study called EDVeRT, conducted in an Emergency Department setting, found that vestibular rehabilitation therapy (VRT) significantly eased dizziness and improved discharge outcomes compared to usual care, marking a promising nonpharmacological intervention.
- In December 2024, Sound Pharmaceuticals announced that its Phase III trial (STOPMD-3) of SPI-1005 (ebselen) met its co-primary efficacy endpoints for improving hearing loss and speech discrimination in patients with Meniere's Disease. MD is a chronic inner ear disorder causing hearing loss, tinnitus, and episodes of vertigo, with no current FDA-approved treatments.
- In September 2024, Spiral Therapeutics announced the successful completion of a Phase1b/2a trial for SPT2101-a longacting dexamethasone gel delivered via their MICS Trademark platform. The results demonstrated significant reductions in vertigo days for Meniere's disease, using a minimally invasive delivery directly to the inner ear.
Vertigo Overview
Vertigo is a type of dizziness characterized by the false sensation that you or your surroundings are spinning or moving, even when there is no actual movement. It is not a condition itself but a symptom of various underlying disorders, most commonly involving the inner ear (vestibular system) or parts of the brain that control balance and spatial orientation. People with vertigo often experience nausea, vomiting, imbalance, and difficulty walking.
Common causes include benign paroxysmal positional vertigo (BPPV), Meniere's disease, vestibular neuritis, and migraines. Less commonly, vertigo may be linked to neurological conditions such as stroke or multiple sclerosis. Treatment depends on the cause and may include vestibular rehabilitation exercises, medications to reduce symptoms, or procedures like the Epley maneuver for BPPV. In some cases, lifestyle changes or surgery may be necessary to manage chronic or severe vertigo.
Find out more about Vertigo medication at https://www.delveinsight.com/report-store/vertigo-pipeline-insight [https://www.delveinsight.com/report-store/vertigo-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=jpr]
Vertigo Treatment Analysis: Drug Profile
AM-125: Auris Medical
Auris Medical is developing AM-125, an intranasal formulation of betahistine, for the treatment of vertigo. Unlike oral betahistine, AM-125 bypasses first-pass metabolism, potentially enhancing both efficacy and tolerability. Betahistine is a small-molecule drug that functions as a partial H1 receptor agonist and H3 receptor antagonist, promoting increased blood flow in the cochlear, vestibular, and cerebral regions. It also supports vestibular compensation and reduces neuronal firing in the vestibular nuclei. The goal of AM-125 is to help restore balance in vertigo patients. The drug is currently in Phase 2 clinical development.
Seliforant: Sensorion
Seliforant (formerly SENS-111) is an investigational histamine H4 receptor antagonist being developed by Sensorion for the symptomatic treatment of vertigo episodes. As the first drug of its class under clinical evaluation for this purpose, Seliforant acts via neuromodulation of sensorineural inner ear cell function. It is a small molecule designed for oral or injectable administration and is currently being tested in a Phase 2 clinical trial.
Learn more about the novel and emerging Vertigo pipeline therapies [https://www.delveinsight.com/report-store/vertigo-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=jpr].
Vertigo Therapeutics Assessment
By Product Type
- Mono
- Combination
- Mono/Combination.
By Stage
- Late-stage products (Phase III)
- Mid-stage products (Phase II)
- Early-stage product (Phase I) along with the details of
- Pre-clinical and Discovery stage candidates
- Discontinued & Inactive candidates
By Route of Administration
- Oral
- Parenteral
- Intravitreal
- Subretinal
- Topical
By Molecule Type
- Monoclonal Antibody
- Peptides
- Polymer
- Small molecule
- Gene therapy
Scope of the Vertigo Pipeline Report
- Coverage: Global
- Key Vertigo Companies: Auris Medical, Sensorion, Apsen Farmaceutica, and others.
- Key Vertigo Pipeline Therapies: AM-125, Seliforant, APSLXR, and others.
Explore detailed insights on drugs used in the treatment of Vertigo here [https://www.delveinsight.com/report-store/vertigo-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=jpr].
Table of Contents
1. Introduction
2. Executive Summary
3. Vertigo Pipeline: Overview
4. Analytical Perspective In-depth Commercial Assessment
5. Vertigo Pipeline Therapeutics
6. Vertigo Pipeline: Late-Stage Products (Phase III)
7. Vertigo Pipeline: Mid-Stage Products (Phase II)
8. Vertigo Pipeline: Early Stage Products (Phase I)
9. Therapeutic Assessment
10. Inactive Products
11. Company-University Collaborations (Licensing/Partnering) Analysis
12. Key Companies
13. Key Products
14. Unmet Needs
15. Market Drivers and Barriers
16. Future Perspectives and Conclusion
17. Analyst Views
18. Appendix
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports Pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform, PharmDelve.
Media Contact
Company Name: DelveInsight
Contact Person: Jatin Vimal
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=vertigo-pipeline-insight-2025-novel-drug-classes-and-central-mechanism-targets-drive-innovation-delveinsight]
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: Nevada
Country: United States
Website: https://www.delveinsight.com/
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Vertigo Pipeline Insight 2025: Novel Drug Classes and Central Mechanism Targets Drive Innovation | DelveInsight here
News-ID: 4137642 • Views: …
More Releases from ABNewswire
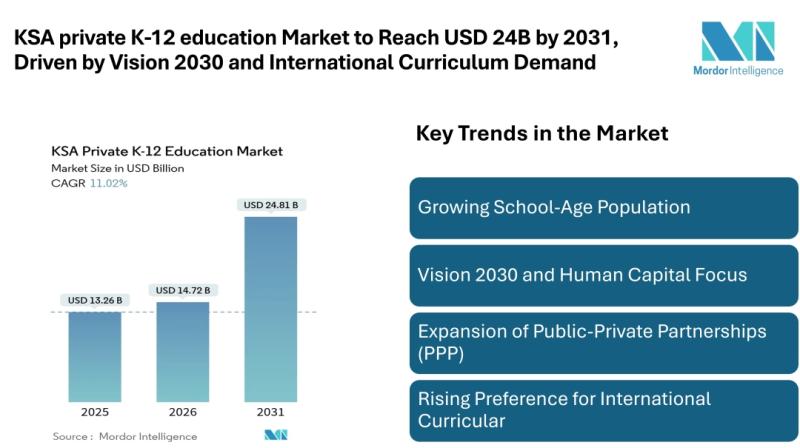
KSA private K-12 education Market to Reach USD 24.81 Billion by 2031, Driven by …
Mordor Intelligence has published a new report on the KSA private K-12 education market, offering a comprehensive analysis of trends, growth drivers, and future projections.
KSA private K-12 education Market Overview
According to Mordor Intelligence, the KSA private K-12 education market size [https://www.mordorintelligence.com/industry-reports/ksa-private-k12-education-market?utm_source=abnewswire] was valued at USD 13.26 billion in 2025 and is estimated to grow from USD 14.72 billion in 2026 to reach USD 24.81 billion by 2031, registering a CAGR…
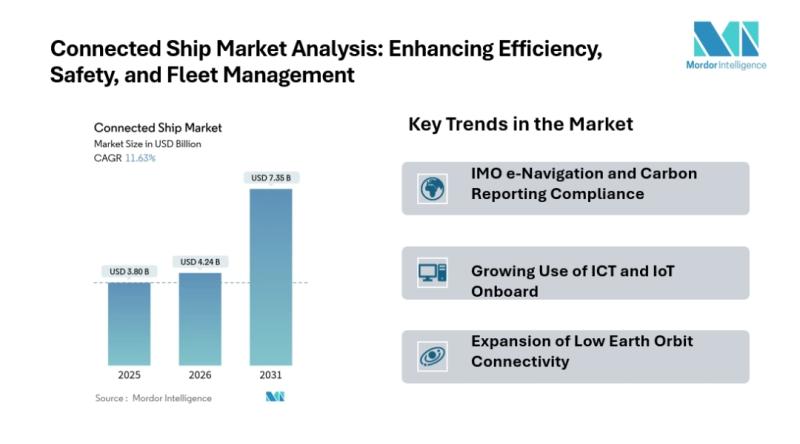
Connected Ship Market to Reach USD 7.35 Billion by 2031, Driven by IMO e-Navigat …
Mordor Intelligence has published a new report on the educational robot market, offering a comprehensive analysis of trends, growth drivers, and future projections.
Connected Ship Market Overview
According to Mordor Intelligence, the connected ship market size is estimated at USD 4.24 billion in 2026, growing from USD 3.80 billion in 2025, and is projected to reach USD 7.35 billion by 2031, expanding at a CAGR of 11.63% during the forecast period. This…

Specialty Chemicals Industry is estimated at $1.26 Trillion in 2026, Forecast to …
Mordor Intelligence has published a new report on the Specialty Chemicals Market, offering a comprehensive analysis of trends, growth drivers, and future projections.
The global [https://www.mordorintelligence.com/industry-reports/specialty-chemicals-market?utm_source=abnewswire] is expected to reach USD 1.54 trillion by 2031, up from USD 1.26 trillion in 2026, growing at a CAGR of 4.09% during the forecast period. Market growth is being driven by large-scale infrastructure projects in Asia-Pacific and GCC countries, rising demand for high-purity chemicals…

Foliar Fertilizer Market Size to Reach USD 30.32 Billion by 2031 - Mordor Intell …
Mordor Intelligence has released a comprehensive report on the foliar fertilizer market, outlining market size expansion, growth catalysts, precision agriculture adoption, and regional opportunities shaping the global industry.
Foliar Fertilizer Market Size and Forecast Outlook
According to a research report by Mordor Intelligence, the global foliar fertilizer market size [https://www.mordorintelligence.com/industry-reports/foliar-fertilizer-market?utm_source=abnewswire] is projected to grow from USD 22.63 billion in 2026 to USD 30.32 billion by 2031, registering a CAGR of 6.03% during…
More Releases for Vertigo
Vertigo Treatment Market to Reach USD 3.42 Billion by 2034
The Global Vertigo Treatment Market is poised for consistent growth as healthcare systems worldwide focus on diagnosing and managing vestibular and balance disorders. According to Exactitude Consultancy, the market, valued at USD 2.18 billion in 2024, is projected to reach USD 3.42 billion by 2034, growing at a CAGR of 4.6 % from 2025 to 2034.
Download Full PDF Sample Copy of Market Report @ https://exactitudeconsultancy.com/request-sample/49856
Rising prevalence of benign paroxysmal positional…
Emerging Trends Influencing The Growth Of The Vertigo Treatment Market: Technolo …
We've updated all our reports with current data on tariff changes, trade developments, and supply chain shifts affecting key industries.
How Big Is the Vertigo Treatment Market Size Expected to Be by 2034?
The market size for vertigo treatment has seen robust growth over the recent years. Its value will escalate from $1.58 billion in 2024 to $1.68 billion in 2025, with a compound annual growth rate (CAGR) of 6.1%. Factors contributing…
Vertigo Treatment Market Trends By 2031: Drug Developments & Technological Advan …
The Vertigo Treatment Market size was at a Significant CAGR of during the forecast period (2024-2031).
The Vertigo Treatment Market report, released by DataM Intelligence, offers comprehensive insights and thorough analysis of major market trends, growth prospects, and emerging challenges. With a strong focus on actionable intelligence, DataM Intelligence equips businesses with the knowledge needed to make strategic decisions and maintain a competitive advantage. Utilizing a blend of qualitative and quantitative…
Vertigo Event Venue Launches a Brand New Website for Clients
Image: https://www.getnews.info/uploads/50c61081fa56e9d8dd4e1ea68a6156ca.jpg
Los Angeles, CA - Vertigo Event Venue proudly announces the launch of its brand new website design, www.vertigo.la [http://www.vertigo.la/], an online portal that redefines the way venue booking is approached in Los Angeles. Nestled in the heart of LA, Vertigo Event Venue in Glendale has been a favored choice for those seeking a blend of modern sophistication and luxurious comfort in event space rentals.
Designed with the user experience in…
Vertigo Treatment Market to Experience Exponential Growth during forecast period
According to a new report published by Allied Market Research, titled,” Vertigo Treatment Market by Type (Peripheral Vertigo and Central Vertigo), Treatment (Medication and Surgery), Route of Administration (Oral and Injectables), End User (Hospitals, Homecare, Specialty Clinics, and Others): Global Opportunity Analysis and Industry Forecast, 2021-2030".
The Global market size of Vertigo Treatment is $XX million in 2020 with XX CAGR, and it is expected to reach $XX million by the…
Grocare Is Offering Herbal Medication For Vertigo Treatment
Based In Pune, India, Grocare is a leading natural solutions provider for chronic diseases. Right from a Kidney Stones, Piles, Gout, Hernia, Varicocele, Tinnitus, Acne, Arthritis, Dental Issue, Urinary disorders, Varicose Vein, Vertigo and Diabetes To Gallbladder Stones, The chronic diseases solutions provider offers effective, research-based affordable medication for it all. Of late, Grocare has emerged as a sought after destination for herbal medications for vertigo treatment.
At Grocare, offering herbal medications…