Press release
Venous Leg Ulcers Clinical Trials, Companies, Therapeutic Assessment, Therapies, Treatment Algorithm, Pipeline Analysis | Reponex Pharmaceuticals, Energenesis Biomedical, MediWound, Solascure limited,
DelveInsight's, "Venous Leg Ulcers - Pipeline Insight, 2025," report provides comprehensive insights about 7+ companies and 7+ pipeline drugs in Venous Leg Ulcers pipeline landscape. It covers the pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.DelveInsight reports that over 7 leading companies are actively engaged in developing more than 7 therapeutic candidates for the treatment of Venous Leg Ulcers.
Venous Leg Ulcers Overview:
Venous leg ulcers (VLUs) are open sores that form between the knee and ankle, primarily due to underlying venous insufficiency. They are the most common type of leg ulcer, making up 60-80% of all cases, with a prevalence of 0.18% to 1% in the general population and up to 4% in those over 65. Around 33-60% of VLUs become chronic, lasting more than six weeks. These ulcers often represent the advanced stage of chronic venous diseases like varicose veins and lipodermatosclerosis.
Several risk factors contribute to their development, including older age, female gender, obesity, trauma, limited mobility, congenital vein issues, DVT, phlebitis, and genetic conditions like factor V Leiden mutation. Clinically, VLUs are typically shallow, irregularly shaped wounds found over bony areas, with a base often made up of granulation tissue and fibrin. Symptoms can include edema, varicose veins, venous dermatitis, and skin changes like lipodermatosclerosis. Recurrence is common, and healing can take weeks to years, with complications such as cellulitis, osteomyelitis, or even malignant transformation in severe cases.
Poor outcomes are generally associated with larger ulcers and longer healing durations. Standard treatments include leg elevation, compression therapy, wound care, and medications such as pentoxifylline or aspirin. For persistent or extensive ulcers, surgical options may be explored.
Request for a detailed insights report on Venous Leg Ulcers pipeline insights @ https://www.delveinsight.com/report-store/venous-leg-ulcers-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr
"Venous Leg Ulcers Pipeline Insight 2025" report by DelveInsight provides a comprehensive analysis of the ongoing clinical development activities and growth prospects across the Venous Leg Ulcers Therapeutics Market.
Key Takeaways from the Venous Leg Ulcers Pipeline Report
*
DelveInsight's report on the Venous Leg Ulcers (VLUs) pipeline highlights a dynamic landscape, with over 7 active companies developing more than 7 potential treatment candidates.
*
Notable players in this space include Reponex Pharmaceuticals, Energenesis Biomedical, MediWound, Solascure Limited, TR Therapeutics, Promore Pharma AB, NovaLead Pharma, RHEACELL, and others, all aiming to enhance the current VLU treatment paradigm.
*
Promising therapies in the pipeline-at various stages of development-include candidates such as ENERGI-F703 and EscharEx.
*
Additionally, a 2023 multicenter randomized self-controlled trial published in the International Wound Journal found that the Geko Trademark device, when combined with standard care, more than doubled healing rates in hard-to-treat VLUs compared to standard care alone.
Venous Leg Ulcers Pipeline Analysis
The report provides insights into:
*
The report provides detailed insights into the key companies that are developing therapies in the Venous Leg Ulcers Market.
*
The report also evaluates different therapeutic candidates segmented into early-stage, mid-stage, and late-stage of development for Venous Leg Ulcers treatment.
*
It analyzes the key companies involved in targeted therapeutics development with respective active and inactive (dormant or discontinued) projects.
*
It navigates the emerging drugs under development based on the stage of development, route of administration, target receptor, monotherapy or combination therapy, a different mechanism of action, and molecular type.
*
Detailed analysis of collaborations (company-company collaborations and company-academia collaborations), licensing agreement, and financing details for future advancement of the Venous Leg Ulcers market.
Download our free sample page report on Venous Leg Ulcers pipeline insights [https://www.delveinsight.com/sample-request/venous-leg-ulcers-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
Venous Leg Ulcers Emerging Drugs
*
ENERGI-F703: Energenesis Biomedical
*
EscharEx: MediWound
Venous Leg Ulcers Companies
Over seven major companies are currently engaged in developing treatments for Venous Leg Ulcers, with Energenesis Biomedical leading the field-its drug candidate has reached the most advanced development stage, currently in Phase II.
DelveInsight's report covers around 7+ products under different phases of clinical development like
*
Late stage products (Phase III)
*
Mid-stage products (Phase II)
*
Early-stage product (Phase I) along with the details of
*
Pre-clinical and Discovery stage candidates
*
Discontinued & Inactive candidates
Venous Leg Ulcers pipeline report provides the therapeutic assessment of the pipeline drugs by the Route of Administration. Products have been categorized under various ROAs such as
*
Intravenous
*
Subcutaneous
*
Oral
*
Intramuscular
Venous Leg Ulcers Products have been categorized under various Molecule types such as
*
Monoclonal antibody
*
Small molecule
*
Peptide
Download Sample Pages to Get an in-depth Assessment of the Emerging Venous Leg Ulcers Therapies and Key Companies: Venous Leg Ulcers Clinical Trials and advancements [https://www.delveinsight.com/report-store/venous-leg-ulcers-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
Venous Leg Ulcers Pipeline Therapeutic Assessment
- Venous Leg Ulcers Assessment by Product Type
- Venous Leg Ulcers By Stage
- Venous Leg Ulcers Assessment by Route of Administration
- Venous Leg Ulcers Assessment by Molecule Type
Download Venous Leg Ulcers Sample report to know in detail about the Venous Leg Ulcers treatment market @ Venous Leg Ulcers Therapeutic Assessment [https://www.delveinsight.com/sample-request/venous-leg-ulcers-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
Table of Content
1. Report Introduction
2. Executive Summary
3. Venous Leg Ulcers Current Treatment Patterns
4. Venous Leg Ulcers - DelveInsight's Analytical Perspective
5. Therapeutic Assessment
6. Venous Leg Ulcers Late-Stage Products (Phase-III)
7. Venous Leg Ulcers Mid-Stage Products (Phase-II)
8. Early Stage Products (Phase-I)
9. Pre-clinical Products and Discovery Stage Products
10. Inactive Products
11. Dormant Products
12. Venous Leg Ulcers Discontinued Products
13. Venous Leg Ulcers Product Profiles
14. Venous Leg Ulcers Key Companies
15. Venous Leg Ulcers Key Products
16. Dormant and Discontinued Products
17. Venous Leg Ulcers Unmet Needs
18. Venous Leg Ulcers Future Perspectives
19. Venous Leg Ulcers Analyst Review
20. Appendix
21. Report Methodology
Request the Sample PDF to Get Detailed Insights About the Venous Leg Ulcers Pipeline Reports Offerings [https://www.delveinsight.com/report-store/venous-leg-ulcers-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports Pharma companies by providing comprehensive end-to-end solutions to improve their performance.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Kritika Rehani
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=venous-leg-ulcers-clinical-trials-companies-therapeutic-assessment-therapies-treatment-algorithm-pipeline-analysis-reponex-pharmaceuticals-energenesis-biomedical-mediwound-solascure-limited]
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: Nevada
Country: United States
Website: https://www.delveinsight.com/
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Venous Leg Ulcers Clinical Trials, Companies, Therapeutic Assessment, Therapies, Treatment Algorithm, Pipeline Analysis | Reponex Pharmaceuticals, Energenesis Biomedical, MediWound, Solascure limited, here
News-ID: 4134164 • Views: …
More Releases from ABNewswire

Windy City Chimney Heroes Announces Expanded Service Areas Across the Greater Ch …
Windy City Chimney Heroes, a long-standing chimney company established in 1997, has expanded its service areas across the Greater Chicago region. The company now serves Chicago, Evanston, Oak Park, Naperville, Schaumburg, Cicero, Skokie, Berwyn, and Arlington Heights. Owner Wesley Cook says the expansion will help meet rising demand for reliable chimney repair and inspection services.
Windy City Chimney Heroes, a trusted chimney repair and maintenance provider founded in 1997, has announced…

Injury 2 Wellness Centers Promotes Wellness Care Through Ongoing Chiropractic Su …
As the year draws to a close and many individuals begin setting health goals for the new year, Injury 2 Wellness Centers is encouraging Georgia residents to prioritize wellness care through consistent chiropractic treatment. Far beyond addressing back or neck pain, chiropractic care can serve as a foundation for long-term physical health, improved function, and overall quality of life.
Decatur, GA - December 10, 2025 - As the year draws to…

Avalon Tree Services Reminds Atlanta Homeowners to Prioritize Winter Tree Care f …
As temperatures drop and trees enter their dormant season, Avalon Tree Services is reminding homeowners across Greater Atlanta that winter is one of the most important times to invest in proper tree care. Cold weather, ice accumulation, and seasonal storms can all take a toll on tree health and stability-making preventative maintenance essential for safety and long-term growth.
Atlanta, GA - December 10, 2025 - As temperatures drop and trees enter…
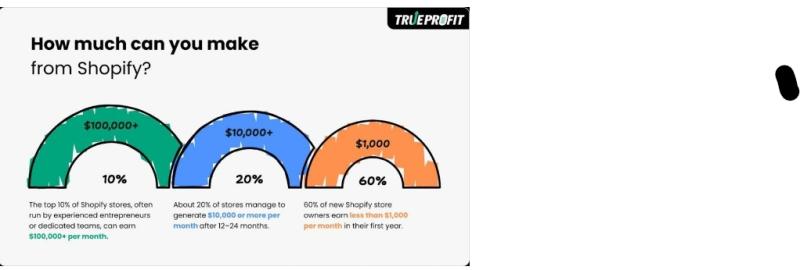
TrueProfit Shares 2026 Guidance for Shopify Startups to Focus on Profit First No …
As Shopify heads into 2026 with millions of active stores worldwide and continued growth in global ecommerce, TrueProfit - a Net Profit Analytics platform for Shopify merchants - is urging new founders to treat profitability as a starting requirement, not an afterthought.
With a low barrier to entry and a mature app ecosystem, Shopify remains one of the easiest ways to launch an online business. But TrueProfit's latest briefing on early-stage…
More Releases for Venous
Key Trends Reshaping the Chronic Venous Occlusions Treatment Market: Introductio …
Use code ONLINE20 to get 20% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
Chronic Venous Occlusions Treatment Market Size Growth Forecast: What to Expect by 2025?
The market dedicated to therapies for chronic venous occlusions has experienced robust expansion lately, projected to increase from a valuation of $6.7 billion in 2024 to $7.15 billion the following year, reflecting a consistent compound annual…
States Venous Stents Market Projected to Witness Expansion with Rising Incidence …
States Venous Stents Market Overview
The States Venous Stents Market is expected to grow from USD 1.3 billion in 2025 to USD 2.4 billion by 2032, registering a CAGR of 8.9%.
Coherent Market Insights is pleased to release its latest States Venous Stents Market research report, offering an in-depth analysis of the U.S. States Venous Stents Market Landscape from 2025 to 2032. This report delivers reliable projections across national and regional levels,…
Venous Stent Market Grows with Rising Cases of Chronic Venous Disorders | Boston …
Venous Stent Market Overview
The venous stent market is anticipated to grow from USD 0.6 billion in 2025 to USD 1.3 billion by 2032, exhibiting a CAGR of 10.3% during the forecast period.
Coherent Market Insights is proud to announce the release of its latest market research report, providing a comprehensive analysis of the Venous Stent Market Landscape from 2025 to 2032. This report delivers precise forecasts across global, regional, and country…
Major Force in the Venous Thromboembolism Market 2025: Impact Of Healthcare Infr …
How Will the Venous Thromboembolism Market Grow, and What Is the Projected Market Size?
Over the past few years, the venous thromboembolism market has experienced a steady increase in its size. It's projected to expand from $2.44 billion in 2024 to $2.55 billion in 2025, rising at a compound annual growth rate (CAGR) of 4.4%. The growth during the historic period can be credited to an increasing elderly population, progress in…
Venous Catheter Market Size 2024 to 2031.
Market Overview and Report Coverage
A venous catheter is a small, flexible tube inserted into a vein for medical purposes such as accessing the bloodstream for medications, fluids, or blood sampling. The venous catheter market is expected to witness significant growth in the coming years, with a projected CAGR of 6.30% during the forecasted period.
The current outlook for the venous catheter market is positive, driven by factors such as…
Increasing Prevalence of Venous Thromboembolism to Augment Growth of the Venous …
Venous thromboembolism (VTE) is the formation of blood clots in the bloodstream. Two main types of venous thromboembolism are pulmonary embolism and deep vein thrombosis. The formation of blood clots in a deep vein is called deep vein thrombosis, while the deep vein thrombosis formed breaks the clot and travels to the lungs, known as pulmonary embolism.
Sample Copy Of Report @ https://www.coherentmarketinsights.com/insight/request-sample/188
Pulmonary embolism occurs in almost one-third of patients…
