Press release
US Biosimilars Market 2025 | FDA Approvals, Oncology Growth & Advanced Biomanufacturing
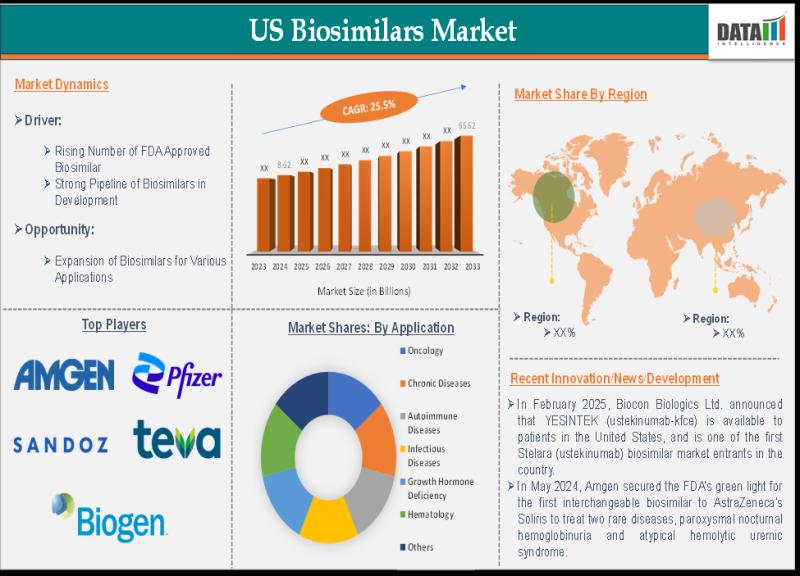
The US Biosimilars Market size reached US$8.62 billion in 2024 and is expected to reach US$65.62 billion by 2033, growing at a CAGR of 25.5% during the forecast period 2025-2033, according to DataM Intelligence. This growth is driven primarily by the expiration of patents for many blockbuster biologics such as Humira, Enbrel, and Remicade, enabling more biosimilar competition to enter the market. Increasing pressure to reduce healthcare costs and rising demand for affordable treatment options for chronic and autoimmune diseases, especially in oncology, further fuel market expansion. Leading players include Pfizer, Amgen, Sandoz (Novartis), Biocon Biologics, Teva Pharmaceutical Industries, Celltrion, Samsung Biologics, Dr. Reddy's Laboratories, and Fresenius Kabi.Download your FREE sample report: https://www.datamintelligence.com/download-sample/us-biosimilars-market?jd
Emerging opportunities in the U.S. biosimilars market include expanding indications into oncology, autoimmune disorders, and endocrinology, advancements in biosimilar manufacturing technologies allowing more complex molecules, and growing collaborations between biosimilar manufacturers and pharmaceutical companies to accelerate development and commercialization. The increasing emphasis on value-based care by payers and the inclusion of biosimilars in formularies enhance adoption rates. The oncology segment is anticipated to grow at the fastest pace due to the high cost of cancer biologics and reimbursement incentives.
Strategic Alliances and Acquisitions Driving Evolution in the US Biosimilars Sector in 2025
1. FDA Approvals and Market Expansion: The FDA approved 10 new biosimilars in Q1 2025 alone, including biosimilars for Stelara (ustekinumab), Actemra (tocilizumab), Xgeva/Prolia (denosumab), Novolog (insulin aspart), and Xolair (omalizumab). Seven of these biosimilars were launched in the US during this period, significantly expanding treatment options and competition, leading to price reductions and improved patient access.
2. Strategic Licensing and Commercial Collaborations: Organon and Henlius entered a strategic alliance to commercialize denosumab biosimilars in the US market. Similarly, Amgen secured exclusive contracts with major pharmacy benefit managers to position their adalimumab biosimilar (Amjevita) and ustekinumab biosimilar (Wezlana) for wider market penetration, driving adoption through pricing and formulary access strategies.
3. Manufacturing Capacity Expansion and Acquisitions: Biosimilar leaders like Celltrion are nearing the acquisition of manufacturing facilities in the US to bolster production capacity and speed market entry. Additionally, companies like Sandoz launched the first interchangeable denosumab biosimilars approved by the FDA in 2025, reinforcing competitive positioning in the immunology biosimilars segment.
Innovative Technological Advances Reshaping the US Biosimilars Industry in 2025
1. Advanced Manufacturing Technologies: Improved bioprocessing, cell line development, and analytical characterization methods enable faster, more cost-effective biosimilar production with high similarity to innovator biologics, including complex recombinant glycosylated proteins.
2. Expanded Therapeutic Applications: While chronic and autoimmune disorders dominate biosimilar indications, oncology is the fastest-growing sector, driven by biosimilar versions of monoclonal antibodies used in cancer treatment.
3. Regulatory and Market Access Innovations: The FDA continues to refine approval pathways, promoting greater biosimilar uptake through educational programs and incentives for healthcare providers and payers, reducing barriers to adoption.
Buy Now & Unlock 360° Market Intelligence: https://www.datamintelligence.com/buy-now-page?report=us-biosimilars-market?jd
The United States leads the biosimilars market globally with the largest revenue share due to a well-established pharmaceutical industry, an advanced regulatory framework, and increasing biosimilar approvals by the FDA. The US benefits from strong government support aimed at reducing healthcare costs through biosimilar adoption, increased payer and provider awareness, and expanded market access supported by competitive pricing. Massive healthcare expenditure in biologics, rising prevalence of chronic diseases (e.g., cancer, autoimmune disorders), and patent expirations of blockbuster biologics create strong demand for biosimilars. The US market saw exponential growth driven by biosimilars for oncology, immunology, and endocrinology indications, with ongoing pipeline expansion expected to sustain momentum.
Request for 2 Days FREE Access:
https://www.datamintelligence.com/reports-subscription?jd
✅ Technology Roadmap Analysis
✅ Sustainability Impact Analysis
✅ KOL / Stakeholder Insights
✅ Pipeline Analysis For Drug Discovery
✅ Positioning, Pricing & Market Access Snapshots
✅ Market Volatility & Emerging Risks Analysis
✅ Competitive Landscape
Have a look at our Subscription Dashboard:
https://www.youtube.com/watch?v=x5oEiqEqTWg?jd
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us:
DataM Intelligence is a leading market research and consulting firm offering end-to-end business solutions from research to strategy consulting. Leveraging industry trends, insights, and extensive data across more than 6,300 reports in over 40 sectors, DataM Intelligence supports over 200 clients across 50+ countries. Our comprehensive research methodologies empower organizations with actionable intelligence to drive growth and innovation.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release US Biosimilars Market 2025 | FDA Approvals, Oncology Growth & Advanced Biomanufacturing here
News-ID: 4131767 • Views: …
More Releases from DataM Intelligence 4Market Research LLP

United States Feed Processing Equipment Market Poised to Reach US$ 73.49 Billion …
Global Feed Processing Equipment Market reached US$ 50.2 billion in 2022 and is expected to reach US$ 73.49 billion by 2031, growing with a CAGR of 4.9% during the forecast period 2024-2031.
Feed processing equipment refers to the machinery and technology used in the production and formulation of animal feed. This equipment includes a range of systems such as grinders, mixers, pelletizers, and extruders that help in the transformation of raw…

Battery Management Integrated Circuit (IC) Market to Grow from US$ 7.4 Billion i …
The Battery Management Integrated Circuit (IC) Market reached US$ 7.4 billion in 2022 and is expected to reach US$ 13.8 billion by 2030, growing with a CAGR of 8.1% from 2024 to 2031.
The Battery Management Integrated Circuit (IC) Market report, published by DataM Intelligence, provides in-depth insights and analysis on key market trends, growth opportunities, and emerging challenges. Committed to delivering actionable intelligence, DataM Intelligence empowers businesses to make…

Solar Panel Market to Reach US$ 642.3 Million by 2031, Growing at a CAGR of 18.5 …
The Solar Panel Market reached US$ 165.2 million in 2022 and is expected to reach US$ 642.3 million by 2031 growing with a CAGR of 18.5% from 2024 to 2031.The Solar Panel Market report, published by DataM Intelligence, provides in-depth insights and analysis on key market trends, growth opportunities, and emerging challenges. Committed to delivering actionable intelligence, DataM Intelligence empowers businesses to make informed decisions and stay ahead of the…

In-Flight Connectivity Market Growth Driven by Streaming, Messaging, and Real-Ti …
➢ In-Flight Connectivity Market - Industry Overview
The In-Flight Connectivity (IFC) Market is rapidly transforming the aviation landscape by enabling real-time internet access, seamless communication, and digital services during flights. Connectivity systems-powered by satellite links, airborne antennas, and onboard network hardware are increasingly adopted by airlines to enhance passenger experience, improve operational efficiency, and unlock new revenue streams.
In-flight connectivity has shifted from a value-added luxury to a critical service that airlines…
More Releases for Biosimilar
Interchangeable Biosimilar Humira Market Share Driven by Biologic Therapy Adopti …
Interchangeable Biosimilar Humira Market
The global market for Interchangeable Biosimilar Humira was valued at US$ million in the year 2024 and is projected to reach a revised size of US$ million by 2031, growing at a CAGR of %during the forecast period
View sample report
https://reports.valuates.com/request/sample/QYRE-Auto-33I15005/Global_Interchangeable_Biosimilar_Humira_Market_Research_Report_2023
The Interchangeable Biosimilar Humira Market is experiencing significant market growth as healthcare providers and patients increasingly adopt biosimilar therapies for autoimmune and inflammatory conditions. Market trends indicate rising…
Key Trend Reshaping the Biosimilar Monoclonal Antibodies Market in 2025: Advance …
What Are the Projections for the Size and Growth Rate of the Biosimilar Monoclonal Antibodies Market?
In recent times, the biosimilar monoclonal antibodies sector has experienced a swift expansion. The market size, which stands at $8.04 billion in 2024, is projected to climb to $9.25 billion in 2025, marking a compound annual growth rate (CAGR) of 15.1%. Factors such as expired patents, an increased understanding of biosimilars, governmental strategies, heightened financial…
Key Trend Reshaping the Biosimilar Monoclonal Antibodies Market in 2025: Advance …
What Are the Projections for the Size and Growth Rate of the Biosimilar Monoclonal Antibodies Market?
In recent times, the biosimilar monoclonal antibodies sector has experienced a swift expansion. The market size, which stands at $8.04 billion in 2024, is projected to climb to $9.25 billion in 2025, marking a compound annual growth rate (CAGR) of 15.1%. Factors such as expired patents, an increased understanding of biosimilars, governmental strategies, heightened financial…
Biosimilar Market Treating More for Less: The Booming Infliximab Biosimilar Mark …
Infliximab Biosimilar Market worth $ XX Million by 2030 - Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Infliximab Biosimilar Market- by Application (Crohn's Disease, Psoriatic Arthritis, Rheumatoid Arthritis, Ulcerative Colitis, Ankylosing Spondylitis, Plaque Psoriasis and Others), End User (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy and Other Direct Distribution Channels), Trends, Industry Competition Analysis, Revenue and Forecast To 2030."
Get…
Biosimilar Monoclonal Antibodies Market
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the " "Global Biosimilar Monoclonal Antibodies Market by Product (infliximab, trastuzumab, rituximab, adalimumab, bevacizumab, cetuximab, ranibizumab, denosumab, eculizumab, and other pipeline products), Indication (oncology, inflammatory & autoimmune disorders, chronic diseases, blood disorders, and other indications), Clinical Trial/Pipeline Analysis, Future Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
The Biosimilar Monoclonal Antibodies Market Size is valued at 5.02…
Infliximab Biosimilar Insight, 2022 | DelveInsight
DelveInsight's, "Infliximab Biosimilar Insight, 2022" report provides comprehensive insights about 35+ companies and 45+ marketed and pipeline drugs in Infliximab Biosimilars landscape. It covers the marketed and pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Interested to know more about the functioning of…