Press release
Intrahepatic Cholangiocarcinoma Pipeline Insight 2025: 20+ Emerging Therapies Target FGFR2, IDH1/2, and Beyond | DelveInsight
ChatGPT said: The intrahepatic cholangiocarcinoma (iCCA) pipeline is growing steadily, with over 18 companies focused on targeted and immunotherapeutic strategies. Key advances include FGFR2 fusions, IDH1/2 mutations, and HER2 amplifications, driving precision oncology in this aggressive liver cancer. Approvals of FGFR inhibitors like Pemazyre and Truseltiq have advanced biomarker-driven second-line treatments.DelveInsight's "Intrahepatic Cholangiocarcinoma - Pipeline Insight, 2025 [https://www.delveinsight.com/report-store/intrahepatic-cholangiocarcinoma-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=jpr]" provides a detailed analysis of the therapeutic development landscape for iCCA, a rare but highly lethal form of bile duct cancer with limited curative options. The majority of patients are diagnosed at an advanced stage, and median survival remains poor, underscoring the need for targeted and immune-based innovations.
Several promising therapies are in late-stage clinical trials, including futibatinib (TAS-120), a next-generation irreversible FGFR inhibitor, and ivosidenib for IDH1-mutant iCCA. Immunotherapies such as durvalumab, nivolumab, and anti-PD-1/CTLA-4 combinations are also being explored, either alone or in synergy with chemotherapy. Novel multi-kinase inhibitors, antibody-drug conjugates, and tumor microenvironment modulators are expanding the treatment armamentarium.
The FDA continues to grant orphan drug designations and breakthrough therapy status to accelerate development in this underserved space. Biomarker testing and molecular profiling are increasingly being integrated into standard diagnostic algorithms, enhancing trial enrollment and personalization of therapy.
As 2025 approaches, the iCCA pipeline is shifting from empirical treatment to targeted, biology-driven approaches-empowered by molecular diagnostics, real-world evidence, and strong academic-industry collaboration. With multiple registrational trials underway, the landscape is poised for significant progress in improving survival and quality of life for patients with this challenging disease.
Interested in learning more about the current treatment landscape and the key drivers shaping the Intrahepatic Cholangiocarcinoma pipeline? Click here [https://www.delveinsight.com/report-store/intrahepatic-cholangiocarcinoma-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=jpr]
Key Takeaways from the Intrahepatic Cholangiocarcinoma Pipeline Report
- DelveInsight's Intrahepatic cholangiocarcinoma pipeline analysis depicts a strong space with 18+ active players working to develop 20+ pipeline drugs for Intrahepatic cholangiocarcinoma treatment.
- The leading Intrahepatic cholangiocarcinoma companies include Zymeworks, Basilea Pharmaceuticals, Medivir AB, Amgen, Kinnate Biopharma, Tyra Biosciences, Trisalus Life Sciences, and others are evaluating their lead assets to improve the Intrahepatic cholangiocarcinoma treatment landscape.
- Key Intrahepatic cholangiocarcinoma pipeline therapies in various stages of development include Zanidatamab, Derazantinib, MIV-818, Bemarituzumab, KIN-3248, TYRA-200, Nelitolimod, and others.
- In April 2025, researchers published results linking molecular resistance mechanisms in FGFR2-altered iCCA to futibatinib treatment, showing genomic correlates and evolving resistance pathways identified through ctDNA profiling and tissue biopsies; the analysis aims to guide future sequencing of FGFR inhibitors.
- In May 2024, the FDA announced the final withdrawal of its accelerated approval for infigratinib (Truseltiq) in FGFR2-positive cholangiocarcinoma. The withdrawal was requested by the sponsor due to challenges in conducting the required confirmatory trial for clinical benefit
Intrahepatic Cholangiocarcinoma Overview
Intrahepatic cholangiocarcinoma (ICC) is a rare and aggressive form of liver cancer that originates in the bile ducts within the liver. It often presents at an advanced stage due to its subtle symptoms, making early diagnosis challenging. ICC is characterized by rapid tumor growth and poor prognosis, with limited treatment options available. Current therapies include surgical resection for eligible patients, chemotherapy, targeted therapies, and emerging immunotherapies aimed at improving outcomes. Research is ongoing to better understand the molecular mechanisms driving ICC to develop more effective treatments.
Find out more about Intrahepatic cholangiocarcinoma medication at https://www.delveinsight.com/report-store/intrahepatic-cholangiocarcinoma-pipeline-insight [https://www.delveinsight.com/report-store/intrahepatic-cholangiocarcinoma-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=jpr]
Intrahepatic Cholangiocarcinoma Treatment Analysis: Drug Profile
Zanidatamab: Zymeworks
Zanidatamab is an investigational bispecific antibody developed using Zymeworks' proprietary Azymetric platform, designed to simultaneously bind two distinct HER2 epitopes through biparatopic binding. This unique approach enables multiple mechanisms of action, including dual HER2 signaling blockade, HER2 protein removal from the cell surface, and immune-mediated cytotoxicity, resulting in promising antitumor effects. Zanidatamab is currently in Phase III clinical trials as a targeted therapy for patients with HER2-expressing solid tumors, including intrahepatic cholangiocarcinoma.
HMPL-453: Hutchmed
HMPL-453 is a novel, highly selective small molecule inhibitor targeting FGFR 1, 2, and 3. Preclinical studies have shown that HMPL-453 offers superior potency and kinase selectivity compared to other FGFR inhibitors, along with a favorable safety profile. It is currently being evaluated in Phase II clinical trials for the treatment of intrahepatic cholangiocarcinoma.
Learn more about the novel and emerging intrahepatic cholangiocarcinoma pipeline therapies [https://www.delveinsight.com/report-store/intrahepatic-cholangiocarcinoma-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=jpr].
Intrahepatic Cholangiocarcinoma Therapeutics Assessment
By Product Type
- Mono
- Combination
- Mono/Combination.
By Stage
- Late-stage products (Phase III)
- Mid-stage products (Phase II)
- Early-stage product (Phase I) along with the details of
- Pre-clinical and Discovery stage candidates
- Discontinued & Inactive candidates
By Route of Administration
- Oral
- Intravenous
- Subcutaneous
- Parenteral
- Topical
By Molecule Type
- Recombinant fusion proteins
- Small molecule
- Monoclonal antibody
- Peptide
- Polymer
- Gene therapy
Scope of the Intrahepatic Cholangiocarcinoma Pipeline Report
- Coverage: Global
- Key Intrahepatic Cholangiocarcinoma Companies: Zymeworks, Basilea Pharmaceuticals, Medivir AB, Amgen, Kinnate Biopharma, Tyra Biosciences, Trisalus Life Sciences, and others.
- Key Intrahepatic Cholangiocarcinoma Pipeline Therapies: Zanidatamab, Derazantinib, MIV-818, Bemarituzumab, KIN-3248, TYRA-200, Nelitolimod, and others.
Explore detailed insights on drugs used in the treatment of intrahepatic cholangiocarcinoma here [https://www.delveinsight.com/report-store/intrahepatic-cholangiocarcinoma-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=jpr].
Table of Contents
1. Introduction
2. Executive Summary
3. Intrahepatic Cholangiocarcinoma Pipeline: Overview
4. Analytical Perspective In-depth Commercial Assessment
5. Intrahepatic Cholangiocarcinoma Pipeline Therapeutics
6. Intrahepatic Cholangiocarcinoma Pipeline: Late-Stage Products (Phase III)
7. Intrahepatic Cholangiocarcinoma Pipeline: Mid-Stage Products (Phase II)
8. Intrahepatic Cholangiocarcinoma Pipeline: Early Stage Products (Phase I)
9. Therapeutic Assessment
10. Inactive Products
11. Company-University Collaborations (Licensing/Partnering) Analysis
12. Key Companies
13. Key Products
14. Unmet Needs
15. Market Drivers and Barriers
16. Future Perspectives and Conclusion
17. Analyst Views
18. Appendix
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports Pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform, PharmDelve.
Media Contact
Company Name: DelveInsight
Contact Person: Jatin Vimal
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=intrahepatic-cholangiocarcinoma-pipeline-insight-2025-20-emerging-therapies-target-fgfr2-idh12-and-beyond-delveinsight]
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: Nevada
Country: United States
Website: https://www.delveinsight.com/
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Intrahepatic Cholangiocarcinoma Pipeline Insight 2025: 20+ Emerging Therapies Target FGFR2, IDH1/2, and Beyond | DelveInsight here
News-ID: 4128509 • Views: …
More Releases from ABNewswire
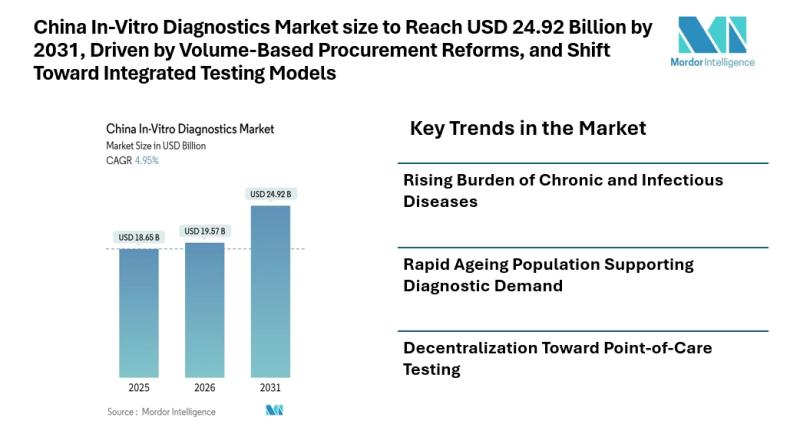
China In-Vitro Diagnostics Market size to Reach USD 24.92 Billion by 2031, Drive …
Mordor Intelligence has published a new report on the china in-vitro diagnostics market, offering a comprehensive analysis of trends, growth drivers, and future projections.
Introduction
According to Mordor Intelligence, the china in-vitro diagnostics market size [https://www.mordorintelligence.com/industry-reports/china-in-vitro-diagnostics-market?utm_source=abnewswire] is projected to reach USD 24.92 billion by 2031, growing from USD 19.57 billion in 2026 at a CAGR of 4.95% during the forecast period. The china in-vitro diagnostics market size reflects steady expansion supported by…

Hyaluronic Acid Market Size to Reach USD 4.07 Billion by 2030 - Mordor Intellige …
Mordor Intelligence has released an in-depth analysis of the hyaluronic acid market, outlining expanding cosmetic, orthopedic, and pharmaceutical applications driving global demand.
Hyaluronic Acid Market Overview
According to Mordor Intelligence, the global hyaluronic acid market size [https://www.mordorintelligence.com/industry-reports/hyaluronic-acid-market?utm_source=abnewswire] reached USD 2.84 billion in 2025 and is projected to grow to USD 4.07 billion by 2030, registering a CAGR of 7.46% during the forecast period.
The strong hyaluronic acid market growth is supported by:
* Increasing…

Scott Bryant Unveils Moon Valley's "Best Value" Listing in Hillcrest East; Signa …
Bryant Real Estate Leverages Data-Driven Performance Metrics to Position New Hillcrest East Property as the Region's Premier Investment Opportunity
PHOENIX, AZ - Scott Bryant, Founder and Team Leader of Bryant Real Estate and a top-performing agent with Keller Williams, has announced the debut of a landmark listing in the Hillcrest East subdivision of Moon Valley. Positioned as "Moon Valley's Best Deal," the property is being introduced at a strategic price point…

Jennifer Rollin Named Best Individual Therapist in Best of Bethesda Awards
Bethesda, MD, USA - Jennifer Rollin, LCSW-C, eating disorder therapist and founder of The Eating Disorder Center, has been named Best Individual Therapist in the 2025 Best of Bethesda Awards. She was selected from among therapists across Montgomery County, Maryland and Upper Northwest Washington, D.C., an honor that reflects both community support and her longstanding commitment to helping individuals recover from eating disorders.
Jennifer Rollin provides eating disorder therapy [https://www.theeatingdisordercenter.com/eatingdisordertherapyrockvilleservices.html] in…
More Releases for Intrahepatic
Intrahepatic Cholangiocarcinoma Pipeline 2025: FDA Updates, Therapy Innovations, …
Las Vega (Nevada), United States //- As per DelveInsight's assessment, globally, Intrahepatic Cholangiocarcinoma pipeline constitutes 18+ key companies continuously working towards developing 20+ Intrahepatic Cholangiocarcinoma treatment therapies, analysis of Clinical Trials, Therapies, Mechanism of Action, Route of Administration, and Developments analyzes DelveInsight.
The Intrahepatic Cholangiocarcinoma Pipeline report embraces in-depth commercial and clinical assessment of the pipeline products from the pre-clinical developmental phase to the marketed phase. The report…
Progressive Familial Intrahepatic Cholestasis (PFIC) Market is projected to reac …
The global Progressive Familial Intrahepatic Cholestasis (PFIC) Market was valued at USD 412 million in 2024 and is projected to reach USD 1.02 billion by 2034, growing at a CAGR of 9.4% during the forecast period (2025-2034). Market growth is driven by rising diagnosis supported by genetic testing, increasing awareness of rare pediatric cholestatic disorders, expanding availability of targeted pharmacologic therapies, and ongoing research into gene-based treatments.
Download Full PDF Sample…
Progressive Familial Intrahepatic Cholestasis (PFIC) Market Growth, Trends, Cons …
Introduction
Progressive Familial Intrahepatic Cholestasis (PFIC) is a group of rare, inherited liver disorders characterized by defective bile secretion, leading to chronic cholestasis, liver damage, and progressive liver failure. PFIC typically manifests in infancy or early childhood and, without intervention, often requires liver transplantation at a young age.
For decades, treatment options were limited to symptomatic relief and surgical interventions. However, with the approval of bile acid transporter inhibitors, alongside the emergence…
Intrahepatic Cholangiocarcinoma Market is expected to reach USD 1.34 billion by …
Intrahepatic cholangiocarcinoma (iCCA) is a rare and aggressive form of bile duct cancer originating within the liver. It accounts for approximately 10-15% of primary liver cancers but has one of the poorest prognoses due to late-stage diagnosis and limited treatment options. Over recent years, genomic profiling has identified actionable mutations-including FGFR2 fusions, IDH1 mutations, and BRAF mutations-leading to the approval of targeted therapies and expanding treatment possibilities.
The iCCA market is…
Transjugular Intrahepatic Portosystemic Shunt (TIPS) Boston Scientific Corporati …
Transjugular Intrahepatic Portosystemic Shunt (TIPS) is a minimally invasive procedure used to treat complications of portal hypertension, such as variceal bleeding and refractory ascites. It involves creating a shunt between the portal and hepatic veins, bypassing the liver to reduce portal pressure. TIPS is performed under fluoroscopic guidance by interventional radiologists. It effectively alleviates symptoms and reduces the risk of complications associated with portal hypertension. TIPS is typically reserved for…
Intrahepatic Cholangiocarcinoma Pipeline Insight and Clinical Trial Phases Repor …
DelveInsight's, "Intrahepatic Cholangiocarcinoma Pipeline Insight, 2023," report provides comprehensive insights about 20+ companies and 20+ pipeline drugs in Intrahepatic Cholangiocarcinoma pipeline landscape. It covers the Intrahepatic Cholangiocarcinoma pipeline drug profiles, including Intrahepatic Cholangiocarcinoma clinical trial and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Key takeaways from the Intrahepatic…