Press release
Omics-Based Clinical Trials Market to Surpass USD 13.56 Billion by 2034, Driven by Precision Medicine and Genomic Innovations
The global omics-based clinical trials market is projected to witness robust growth over the next decade, increasing from approximately USD 6.46 billion in 2024 to around USD 13.56 billion by 2034. This impressive expansion reflects a compound annual growth rate (CAGR) of about 7.70% between 2025 and 2034, driven by the growing demand for precision medicine, advancements in genomic technologies, and the rising importance of personalized therapeutics.Access key findings and insights from our Report in this sample -https://www.zionmarketresearch.com/sample/omics-based-clinical-trials-market
What are Omics-Based Clinical Trials?
Omics-based clinical trials integrate data from genomics, proteomics, transcriptomics, metabolomics, and other "omics" fields to gain deeper insights into disease mechanisms and treatment responses. These trials enable a more comprehensive understanding of individual patient profiles, helping researchers and clinicians tailor therapies with greater accuracy and effectiveness.
Omics-Based Clinical Trials Market: Competitive Analysis
The global omics-based clinical trials market is led by players like:
Novo Nordisk
Parexel International Corporation
GSK (GlaxoSmithKline)
Pharmaceutical Product Development (PPD)
Moderna
Charles River Laboratory
Freenome
Eli Lilly and Company
ICON plc
Genomics
Pfizer Inc.
BioNTech
SGS SA
Rebus Bio
Covance Inc.
Key Market Drivers:
Precision Medicine Revolution:
The global shift towards individualized treatment strategies is fueling the demand for omics-based trials, especially in oncology, rare diseases, and chronic conditions.
Advancements in Sequencing and Bioinformatics:
Cost-effective next-generation sequencing (NGS), AI-powered data analytics, and improved bioinformatics platforms are enhancing the scalability and reliability of omics studies.
Growing Pharma and Biotech Investments:
Pharmaceutical and biotech companies are increasingly investing in omics technologies to accelerate drug development, identify novel biomarkers, and reduce trial attrition rates.
Supportive Regulatory Environment:
Regulatory bodies like the FDA and EMA are recognizing the value of omics data in drug evaluation and are creating frameworks to facilitate their integration into clinical trials.
Regional Insights:
North America dominates the market, with strong research infrastructure, government funding, and a high number of biotech startups.
Europe follows closely, driven by EU-led genomics initiatives and collaborations between academic and commercial sectors.
Asia-Pacific is emerging as a promising region, especially with growing clinical research hubs in China, India, and South Korea.
Access our report for a comprehensive look at key insights -https://www.zionmarketresearch.com/report/omics-based-clinical-trials-market
Applications & Trial Types:
Most omics-based trials are focused on oncology, but there's rising interest in neurology, cardiology, and infectious diseases.
Trials incorporating multi-omics approaches are gaining traction for their holistic disease profiling capabilities.
Future Outlook:
As healthcare increasingly embraces precision and data-driven strategies, omics-based clinical trials will play a pivotal role in reshaping how new therapies are developed and validated. Stakeholders across the pharmaceutical, biotech, and healthcare sectors are expected to accelerate their adoption of omics platforms to enhance therapeutic outcomes and reduce time-to-market.
More Trending Reports by Zion Market Research -
Reactive Diluents Market-https://www.zionmarketresearch.com/report/reactive-diluents-market
Calcium Hypochlorite Market-https://www.zionmarketresearch.com/report/calcium-hypochlorite-market
Dicyandiamide Market-https://www.zionmarketresearch.com/report/dicyandiamide-market
Spray Adhesives Market-https://www.zionmarketresearch.com/report/spray-adhesives-market
Polyaluminium Chloride Market-https://www.zionmarketresearch.com/report/polyaluminum-chloride-market
Asia Pacific Office
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1-855-465-4651
Email: sales@zionmarketresearch.com
Zion Market Research is an obligated company. We create futuristic, cutting edge, informative reports ranging from industry reports, company reports to country reports. We provide our clients not only with market statistics unveiled by avowed private publishers and public organizations but also with vogue and newest industry reports along with pre-eminent and niche company profiles. Our database of market research reports comprises a wide variety of reports from cardinal industries. Our database is been updated constantly in order to fulfill our clients with prompt and direct online access to our database. Keeping in mind the client's needs, we have included expert insights on global industries, products, and market trends in this database. Last but not the least, we make it our duty to ensure the success of clients connected to us-after all-if you do well, a little of the light shines on us.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Omics-Based Clinical Trials Market to Surpass USD 13.56 Billion by 2034, Driven by Precision Medicine and Genomic Innovations here
News-ID: 4114160 • Views: …
More Releases from Zion Market Research

Halal Food Market to Reach USD 16.84 Billion by 2034, Expanding at 18.04% CAGR
The global halal food market, valued at USD 3.21 billion in 2024, is projected to reach USD 16.84 billion by 2034 at a robust CAGR of 18.04%. This extraordinary growth is fueled by a rapidly rising global Muslim population, increasing demand for certified halal-compliant food, expanding global halal trade networks, and the emergence of halal as a trusted, premium, ethical, and hygienic food label even for non-Muslim consumers.
Key Market Highlights
Metrics Insight
2024…
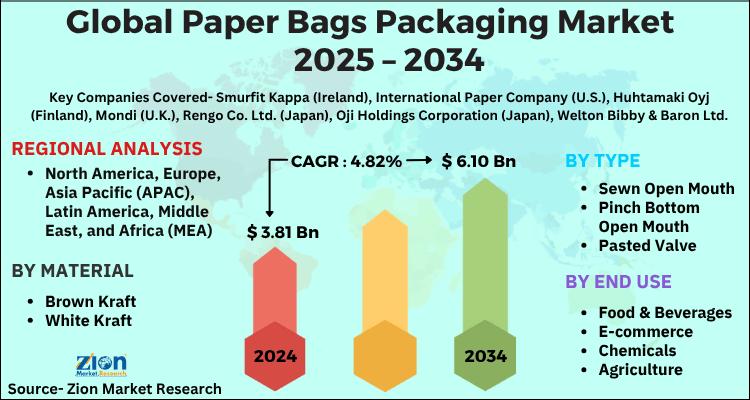
Paper Bags Packaging Market to Reach USD 6.10 Billion by 2034, Expanding at 4.82 …
The global paper bags packaging market, valued at USD 3.81 billion in 2024, is projected to reach USD 6.10 billion by 2034, growing at a 4.82% CAGR between 2025 and 2034. The market is gaining momentum on the back of sustainability mandates, stringent global regulations against single-use plastic, rising consumer environmental consciousness, and the rapid expansion of e-commerce and foodservice industries adopting recyclable packaging.
Key Market Highlights
Indicator Insight
2024 Market Value USD 3.81 Billion
2034…
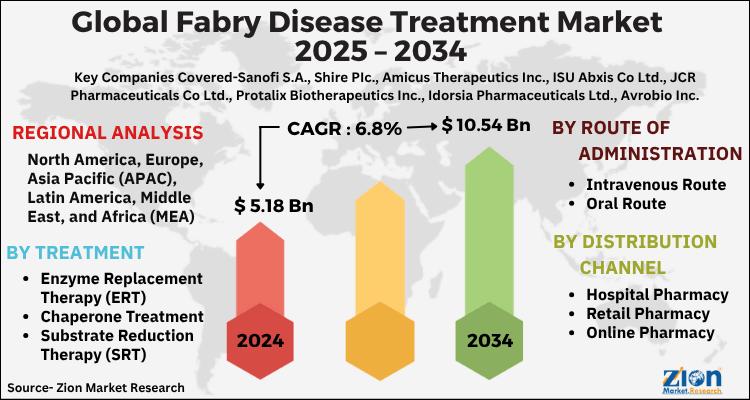
Fabry Disease Treatment Market to Reach USD 10.54 Billion by 2034, Expanding at …
The global Fabry disease treatment market, valued at USD 5.18 billion in 2024, is projected to reach USD 10.54 billion by 2034, growing at a 6.8% CAGR (2025-2034). Market momentum is driven by rising disease awareness and diagnosis, expanding enzyme replacement therapy (ERT) utilization, progress in chaperone and substrate reduction therapies (SRT), and an advancing pipeline in gene and next-generation ERTs. Persistent unmet need-stemming from organ involvement (renal, cardiac, cerebrovascular),…
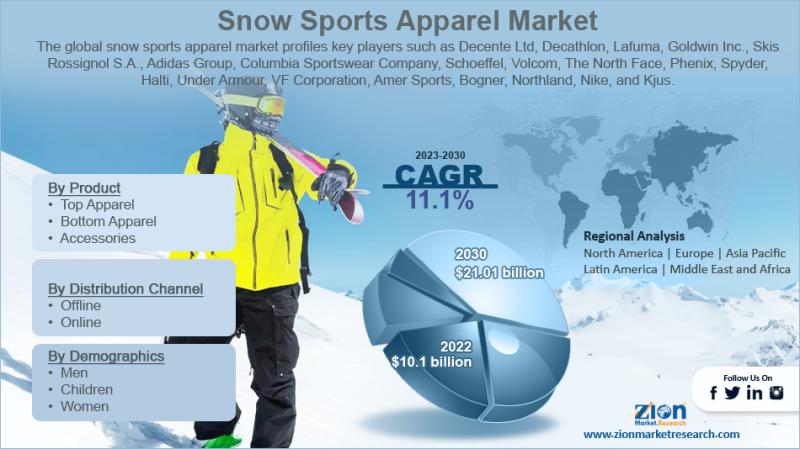
Snow Sports Apparel Market to Reach USD 5.37 Billion by 2034, Expanding at 7.3% …
The global snow sports apparel market, valued at USD 2.65 billion in 2024, is projected to reach USD 5.37 billion by 2034, growing at a 7.3% CAGR (2025-2034). Growth is driven by the rising popularity of winter sports and outdoor recreation, fabric and garment-tech innovations (breathability, waterproofing, thermal regulation), and the accelerating role of e-commerce, social media, and athlete-led branding in discovery and conversion.
Strategic Market Insights & Key Performance Indicators
2024…
More Releases for Trial
Clinical Trial Investigative Site Network Market Clinical Trial Investigative Si …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trial Investigative Site Network Market - (By Therapeutic Areas (Oncology, Cardiology, CNS, Pain Management, Endocrine, Others), By Phase (Phase I, Phase II, Phase III, Phase IV), By End-use (Sponsor, CRO)), Trends, Industry Competition Analysis, Revenue and Forecast To 2034."
According to the latest research by InsightAce Analytic, the Global Clinical Trial Investigative Site Network Market…
Transformative Trends Impacting the Electronic Trial Master File (eTMF) Systems …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Electronic Trial Master File (eTMF) Systems Market Size By 2025?
The market size of the electronic trial master file (eTMF) systems has experienced fast growth over recent years. The market is projected to increase from $1.36 billion in 2024 to $1.55 billion in 2025, with…
Transformative Trends Impacting the Electronic Trial Master File (eTMF) Systems …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Electronic Trial Master File (eTMF) Systems Market Size By 2025?
The market size of the electronic trial master file (eTMF) systems has experienced fast growth over recent years. The market is projected to increase from $1.36 billion in 2024 to $1.55 billion in 2025, with…
Clinical Trial Imaging market
The Clinical Trial Imaging market crossed the US$ 1.09 billion mark in 2022 and is expected to hit US$ 1.94 billion by 2030, recording a CAGR of 7.5% during the forecast period.
Rising R&D spending, a rapidly growing pharmaceutical industry, and an increase in the number of contract research organizations are some of the major factors driving the market's growth. There has been an increase in pharmaceutical companies due to the…
Clinical Trial Logistics
Clinical Trial Logistics
16th to 17th May 2011, Marriott Regents Park, London, United Kingdom.
It currently costs just over £500 million ($800 million) to bring a new chemical to market and development timelines continue to fall in the 10-15 year range. A key reason for high R&D costs is due to logistical failures including failure to recruit patients on time. A way to avoid this is to move clinical trials…
Clinical Trial Logistics
Announcing SMi's 5th annual…
Clinical Trial Logistics conference
16th and 17th May 2011, Central London, UK
www.smi-online.co.uk/2011logistics-london6.asp
It currently costs just over £500 million ($800 million) to bring a new chemical to market and development timelines continue to fall in the 10-15 year range. A key reason for high R&D costs is due to logistical failures including failure to recruit patients on time. A way to avoid this is to move clinical…
