Press release
Pharmacovigilance Market Set for 8.8% CAGR Growth Through 2035
The worldwide pharmacovigilance market is expected to increase at a compound annual growth rate (CAGR) of 8.8% from its estimated valuation of USD 9025.2 million in 2025 to USD 20,977.1 million by 2035. The growing prevalence of chronic illnesses, the growing incidence of adverse drug reactions (ADRs), and the growing pharmaceutical business are the main drivers of this rise.Get Sample Report: - https://www.futuremarketinsights.com/reports/sample/rep-gb-1107
As the pharmaceutical industry continues its rapid evolution, the importance of monitoring drug safety has never been more critical. Pharmacovigilance, a cornerstone of post-marketing surveillance, ensures that medications on the market remain safe and effective for consumers. With increasing regulatory scrutiny, a growing global patient population, and the rise of personalized medicine, the pharmacovigilance market is poised for significant transformation from 2025 to 2035.
What Is Pharmacovigilance?
Pharmacovigilance refers to the science and activities involved in the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problems. It plays a pivotal role in protecting public health by identifying previously unrecognized adverse reactions, evaluating the risk-benefit balance of drugs, and communicating findings to stakeholders.
This function extends beyond mere compliance-it serves as a proactive mechanism for healthcare systems to ensure long-term drug safety, foster trust in pharmaceutical innovation, and minimize healthcare costs associated with adverse drug reactions (ADRs).
Market Outlook (2025-2035)
The pharmacovigilance market is expected to undergo considerable changes during the 2025-2035 decade. With expanding global healthcare access, digital transformation in clinical operations, and tighter regulatory requirements, the need for efficient pharmacovigilance systems is escalating.
Technological advancements such as artificial intelligence (AI), real-world data (RWD) analytics, and blockchain are reshaping how drug safety data is collected, processed, and reported. Simultaneously, the scope of pharmacovigilance is expanding from traditional small-molecule drugs to include biologics, biosimilars, vaccines, and gene therapies.
In this context, pharmacovigilance will no longer be an isolated department within pharmaceutical companies but an integral component of the entire drug development and post-marketing lifecycle.
Market Overview
Traditionally dominated by manual processes and siloed data systems, the pharmacovigilance industry is transitioning into a more agile and integrated domain. Outsourcing pharmacovigilance services to specialized vendors is becoming increasingly common, especially among mid-sized and smaller pharmaceutical companies looking to reduce operational costs.
Geographically, while North America and Europe continue to lead the market in regulatory sophistication and infrastructure, emerging economies in Asia-Pacific and Latin America are becoming increasingly significant due to their rising pharmaceutical manufacturing activities and evolving regulatory frameworks.
The market can be broadly categorized into different segments based on service type (e.g., spontaneous reporting, medical information, case processing, signal detection), clinical phase (preclinical, Phase I-IV), and end-user type (pharma companies, CROs, and regulatory authorities).
Download Brochure PDF:- https://www.futuremarketinsights.com/reports/brochure/rep-gb-1107
Growth Drivers
Several key factors are contributing to the robust growth of the pharmacovigilance market:
Increased Drug Approvals
The surge in global drug approvals, particularly in oncology, immunotherapy, and rare diseases, necessitates a parallel investment in post-market safety monitoring.
Stringent Regulatory Norms
Agencies like the FDA, EMA, and PMDA have heightened expectations around drug safety reporting, which compels companies to invest in advanced pharmacovigilance systems.
Rise in ADRs
Adverse drug reactions are a growing concern, prompting both healthcare providers and governments to prioritize pharmacovigilance programs.
Digital Transformation
The integration of AI and machine learning into pharmacovigilance has enhanced the efficiency of signal detection, case triage, and compliance reporting.
Globalization of Clinical Trials
With clinical trials increasingly being conducted across diverse geographies, pharmacovigilance systems must adapt to heterogeneous regulatory environments and patient populations.
Demand Landscape
The demand for robust pharmacovigilance services is rising across various stakeholders in the healthcare ecosystem:
• Pharmaceutical Companies: With increasing pipeline diversity, companies are relying on pharmacovigilance to manage risks and maintain brand integrity.
• Regulatory Authorities: Government bodies are pushing for increased transparency and data-sharing, requiring real-time pharmacovigilance capabilities.
• Patients and Healthcare Providers: A more informed patient population is driving higher expectations for drug safety and transparency.
Key Trends (2025-2035)
AI-Powered Automation
AI and natural language processing (NLP) will play a larger role in case processing, literature screening, and pattern recognition, reducing manual workloads.
Cloud-Based Solutions
Cloud-native pharmacovigilance platforms will offer scalability, security, and real-time collaboration between global stakeholders.
Real-World Evidence (RWE) Integration
Linking pharmacovigilance with real-world data from EHRs, wearables, and patient registries will refine risk assessments and enable proactive interventions.
Pharmacogenomics
Understanding how genetic variation affects drug response will open new dimensions in personalized safety monitoring.
Blockchain for Data Integrity
Blockchain could revolutionize data traceability and compliance by ensuring secure, immutable records for audits and inspections.
Regulatory Harmonization
Efforts by global regulatory bodies to align pharmacovigilance standards will simplify cross-border drug safety reporting.
Competitive Landscape
The pharmacovigilance industry is marked by intense competition among service providers, technology vendors, and pharma giants. Major players are continuously innovating to enhance their service portfolios, integrate automation, and offer end-to-end safety solutions.
Large pharmaceutical firms often maintain in-house pharmacovigilance departments but increasingly partner with Contract Research Organizations (CROs) for operational efficiency. Meanwhile, specialized pharmacovigilance firms offer focused services such as case management, signal detection, and risk mitigation strategies.
Startups are also disrupting the space by offering AI-based platforms tailored for smaller pharma companies, accelerating drug safety workflows without the need for large teams.
Mergers, acquisitions, and partnerships are frequent, driven by the need to gain technological advantage or access new geographies. Companies that can adapt to regulatory updates, handle large volumes of data securely, and provide real-time insights will likely dominate the future market.
Click Here to Purchase the Report:- https://www.futuremarketinsights.com/checkout/1107
Contact Us:
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware - 19713, USA
T: +1-347-918-3531
For Sales Enquiries: sales@futuremarketinsights.com
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs | YouTube
About Future Market Insights (FMI)
Future Market Insights, Inc. (ESOMAR certified, recipient of the Stevie Award, and a member of the Greater New York Chamber of Commerce) offers profound insights into the driving factors that are boosting demand in the market. FMI stands as the leading global provider of market intelligence, advisory services, consulting, and events for the Packaging, Food and Beverage, Consumer Technology, Healthcare, Industrial, and Chemicals markets. With a vast team of over 400 analystsworldwide, FMI provides global, regional, and local expertise on diverse domains and industry trends across more than 110 countries.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Pharmacovigilance Market Set for 8.8% CAGR Growth Through 2035 here
News-ID: 4113128 • Views: …
More Releases from Future Market Insights
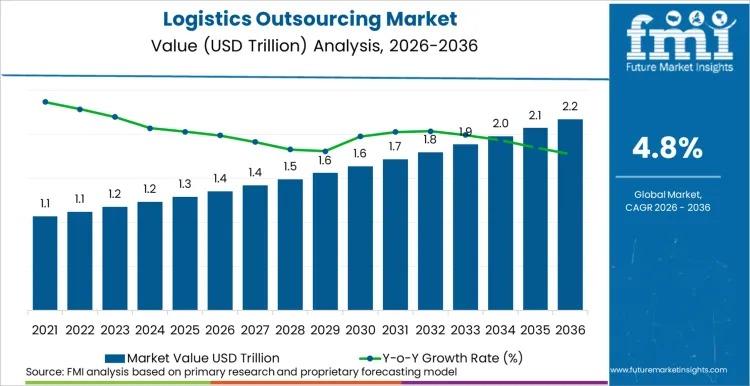
Global Logistics Outsourcing Market to Reach USD 2.2 Trillion by 2036 at 4.8% CA …
The global Logistics Outsourcing Market is projected to expand from USD 1.4 trillion in 2026 to USD 2.2 trillion by 2036, registering a CAGR of 4.8% during the forecast period. According to Future Market Insights (FMI), enterprises are accelerating outsourcing strategies to enhance supply chain resilience, digital transparency, and operational flexibility in an increasingly volatile global trade environment.
Demand dynamics are heavily influenced by the need for end-to-end visibility, omnichannel fulfillment…
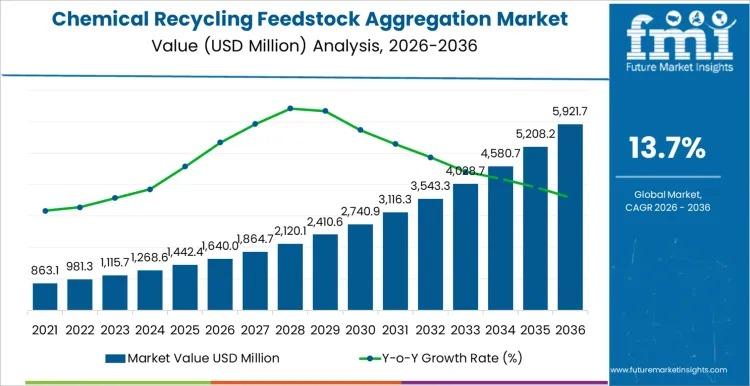
Chemical Recycling Feedstock Aggregation Market to Reach USD 5,921.7 million by …
The global Chemical Recycling Feedstock Aggregation Market is projected to grow from USD 1,640.0 million in 2026 to USD 5,921.7 million by 2036, registering a CAGR of 13.7%. The expansion reflects structural scaling of chemical recycling plants that depend on consistent, specification-aligned plastic waste streams rather than fragmented sourcing models.
As pyrolysis and depolymerization capacities expand worldwide, aggregators are investing in centralized hubs that integrate pre-sorting, blending, contamination control, and logistics…
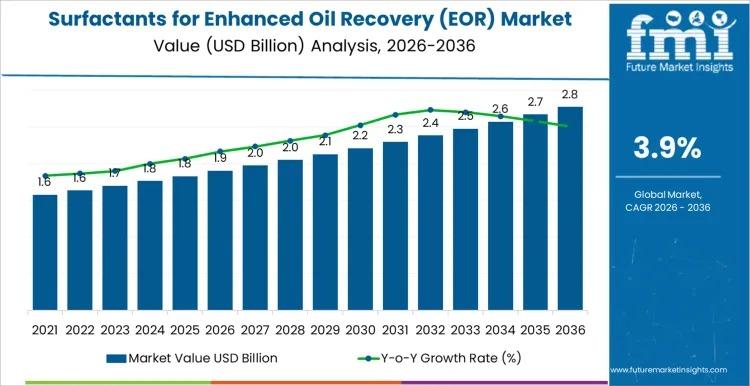
Global Surfactants for Enhanced Oil Recovery Market to Reach USD 2.9 Billion by …
The global Surfactants for Enhanced Oil Recovery (EOR) Market is projected to grow from USD 1.9 billion in 2026 to USD 2.9 billion by 2036, registering a CAGR of 3.85%. Market expansion is closely tied to mature oilfield economics, where incremental recovery gains justify chemical investment. Rather than broad upstream expansion, growth is concentrated in technically validated, project-specific deployments.
Long evaluation timelines, pilot testing requirements, and reservoir heterogeneity slow rapid scale-up,…
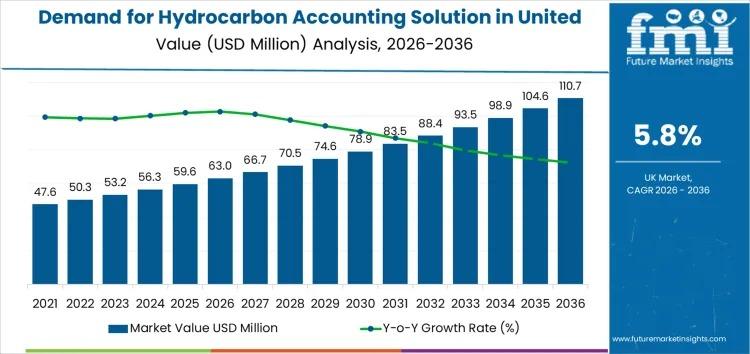
UK Hydrocarbon Accounting Solution Market to Reach USD 110.7 Mn by 2036 at 5.8% …
The Demand for Hydrocarbon Accounting Solution in United Kingdom is projected to expand from USD 63.0 million in 2026 to USD 110.7 million by 2036, registering a CAGR of 5.8% over the forecast period. The market's growth trajectory reflects accelerating regulatory oversight, digital oilfield deployment, and enterprise-level data governance initiatives across upstream and midstream operations.
As operators confront tightening volumetric reporting standards and emissions accountability frameworks, hydrocarbon accounting solutions are becoming…
More Releases for Pharmacovigilance
Top Pharmacovigilance Companies Analysis By 2031
The Pharmacovigilance Market is expected to register a CAGR of 6.6% from 2025 to 2031, with a market size expanding from US$ XX million in 2024 to US$ XX Million by 2031.
Download PDF Copy @ https://www.theinsightpartners.com/sample/TIPRE00003127?utm_source=OpenPR&utm_medium=10379
The List of Companies
• #Accentures
• Bristol-Myers Squibb Company
• Linical Accelovance
• Cognizant
• Covance Inc.
• F. Hoffmann-La Roche Ltd.
• GlaxoSmithKline plc.
• ICON plc
• Capgemini (IGATE Corporation)
Clinical…
Pharmacovigilance - Scope and Research Methodology
The Pharmacovigilance Market is expected to register a CAGR of 6.6% from 2025 to 2031, with a market size expanding from US$ XX million in 2024 to US$ XX Million by 2031.
The Pharmacovigilance Market report covers analysis by Clinical Trial Phase (Pre-Clinical, Phase I, Phase II, Phase III, and Phase IV), Service Provider (In-House and Contract Outsourcing), Type of Method (Spontaneous Reporting, Intensified ADR Reporting, Targeted Spontaneous Reporting, Cohort Event…
Pharmacovigilance World 2025 Conference & Expo
We are delighted to welcome you to the Pharmacovigilance World 2025, and we are confident that your active participation will contribute to the advancement of drug safety practices. Together, let us strive towards a safer and more vigilant healthcare system that prioritizes patient well-being and ensures the continued benefit of medications worldwide.
As medical science advances, so does our understanding of drug safety and the need for vigilance when it comes…
Top Factor Driving Pharmacovigilance Market Growth in 2025: Research And Develop …
How Are the key drivers contributing to the expansion of the pharmacovigilance market?
The escalation in research and development undertakings stimulates growth in the pharmacovigilance market. Pharmaceutical organizations can create novel and superior drugs through enhanced safety profiles by allocating resources to R&D. The intensive testing in preclinical and clinical stages during the drug development protocol allows early recognition of potential safety issues, paving the way for adequate risk reduction approaches.…
Monitoring Medication Safety with Pharmacovigilance
Pharmacovigilance (PV) is defined as the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other drug-related problem. Pharmacovigilance plays a significant role in pharmaceutical and biotechnological sectors in designing of drugs and their interactions. The pharmacovigilance involves collecting information from healthcare providers and patients to know about the hazards associated with medications.
Download Sample PDF at: https://www.theinsightpartners.com/sample/TIPRE00003127?utm_source=OpnePR&utm_medium=10776
Increasing cases of adverse drug reactions…
Pharmacovigilance Market Opportunity Analysis by 2028
Pharmacovigilance Market: Introduction
According to the report, the global pharmacovigilance market was valued at US$ 6.1 Bn in 2020 and is projected to expand at a CAGR of 8.8% from 2021 to 2028. Pharmacovigilance activities are defined as science used for detection, assessment, understanding, and prevention of adverse effects of drugs and vaccines. Drugs and vaccines go through rigorous testing in the clinical trials to check their safety and efficacy before…
