Press release
Recurrent Glioblastoma Pipeline Appears Promising With 50+ Leading Biotech and Pharma Companies Driving Innovation in the Therapeutics Segment | DelveInsight
DelveInsight's, "Recurrent Glioblastoma Pipeline Insight 2025" report provides comprehensive insights about 50+ companies and 50+ pipeline drugs in Recurrent Glioblastoma pipeline landscape. It covers the Recurrent Glioblastoma pipeline drug profiles, including clinical and nonclinical stage products. It also covers the Recurrent Glioblastoma pipeline therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.Explore our latest breakthroughs in Recurrent Glioblastoma Research. Learn more about our innovative pipeline today! @ Recurrent Glioblastoma Pipeline Outlook [https://www.delveinsight.com/sample-request/recurrent-glioblastoma-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Key Takeaways from the Recurrent Glioblastoma Pipeline Report
* In July 2025, CarThera announced a study is protected from any toxic or inflammatory molecule by the blood-brain barrier (BBB). This physical barrier is located at the level of the blood vessel walls. Because of these barrier properties, the blood vessels are also impermeable to the passage of therapeutic molecules from the blood to the brain. The development of effective treatments against glioblastoma is thus limited due to the BBB that prevents most drugs injected in the bloodstream from getting into brain tissue where the tumour is seated.
* DelveInsight's Recurrent Glioblastoma pipeline report depicts a robust space with 50+ active players working to develop 50+ pipeline therapies for Recurrent Glioblastoma treatment.
* The leading Recurrent Glioblastoma Companies such as Ascletis, Genexine, PharmAbcine, VAXIMM AG, WPD Pharmaceuticals, Accendatech USA Inc., Midatech Ltd, MediciNova, Kadmon Corporation, LLC, Istari Oncology, Inc., Bristol-Myers Squibb, Novartis, Jiangsu Hengrui Medicine, Peloton Therapeutics, Inc., Karyopharm Therapeutics, VBL Therapeutics, Nerviano Medical Sciences, Acerta Pharma BV, Basilea Pharmaceutica, DNAtrix, Inc., NanoPharmaceuticals LLC, Erasca, Inc., Oblato, Inc., OX2 Therapeutics, Crimson Biopharm Inc., Merck Sharp & Dohme LLC, Transgene, CANbridge Life Sciences Ltd., Eli Lilly and Company, Arcus Biosciences, Inc., Incyte Corporation, BerGenBio ASA, Istari Oncology, Inc., and Chimerix and others.
* Promising Recurrent Glioblastoma Pipeline Therapies such as Pembrolizumab, Olaparib, Temozolomide, BIBF1120, Chemotherapy, GX-I7, Bevacizumab, Bevacizumab, TTAC-0001, Cediranib and others.
Stay informed about the cutting-edge advancements in Recurrent Glioblastoma treatments. Download for updates and be a part of the revolution in care @ Recurrent Glioblastoma Clinical Trials Assessment [https://www.delveinsight.com/sample-request/recurrent-glioblastoma-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Recurrent Glioblastoma Emerging Drugs Profile
* ASC40: Ascletis
ASC40 is an oral, selective inhibitor of fatty acid synthase (FASN), a key enzyme which regulates de novo lipogenesis (DNL). ASC40 inhibits energy supply and disturbs membrane phospholipid composition of tumor cells by blocking de novo lipogenesis. In January 2022, Ascletis Pharma Inc. announced the dosing of the first patient in the Phase III registration clinical trial of ASC40 combined with bevacizumab for treatment of recurrent glioblastoma (rGBM). The Phase II study, completed in the U.S., in patients with rGBM has shown that the objective response rate (ORR) for ASC40 plus Bevacizumab treatment was 65% including a complete response (CR) of 20% and a partial response (PR) of 45%.
* GX-I7: Genexine
GX-I7 is a long-acting human IL-7 which is essential for homeostatic T cell proliferation and improves lymphopenia, typically induced by chemotherapy or radiation therapy. The safety has been proved via phase I clinical trial in healthy volunteers and phase Ib and Ib/2 Clinical trials are being conducted to evaluate the safety, tolerability, pharmacokinetics/pharmacodynamics and efficacy.
* Olinvacimab: PharmAbcine
Olinvacimab is an anti-angiogenic antibody that neutralizes the VEGF/VEGFR2 pathway, thus inhibiting tumor growth and metastasis. It blocks the binding of all VEGFR ligands such as VEGF-A, VEGF-C and VEGF-D to VEGFR2. To gain nutrients and oxygen needed for growth, tumor cells release these VEGF ligands which promote angiogenesis (a formation of new blood vessels) that will enhance tumor blood supply. Binding of olinvacimab to VEGFR2 will result in the inhibition of VEGF-mediated tumor angiogenesis.
* VXM01: VAXIMM AG
VXM01 is an oral T-cell immunotherapy that is designed to activate T-cells to attack the tumor vasculature and, in several tumor types, attack cancer cells directly. VXM01 carries the vascular endothelial growth factor receptor-2 (VEGFR2), which is highly overexpressed on the tumor vasculature and on certain cancer cells as the target gene. The active, T-cell-mediated destruction of tumor vasculature cells leads to an increased infiltration of various immune cells into tumor tissue (inflammation). In preclinical studies, a murine analog VXM01 vaccine showed broad anti-tumor activity in different tumor types. This activity was linked to a VEGFR2-specific T-cell response and was accompanied by the destruction of the tumor vasculature and increased immune cell infiltration. A Phase I/II trial evaluating VXM01 in combination with avelumab, a human anti-PD-L1 antibody, for the treatment of glioblastoma is ongoing. The trial is part of a collaboration agreement with Merck KGaA, Darmstadt, Germany and Pfizer Inc. VXM01 has received orphan designation from the European Commission and from the US Food and Drug Administration (FDA) for the treatment of glioblastoma.
The Recurrent Glioblastoma Pipeline Report Provides Insights into
* The report provides detailed insights about companies that are developing therapies for the treatment of Recurrent Glioblastoma with aggregate therapies developed by each company for the same.
* It accesses the Different therapeutic candidates segmented into early-stage, mid-stage, and late-stage of development for Recurrent Glioblastoma Treatment.
* Recurrent Glioblastoma Companies are involved in targeted therapeutics development with respective active and inactive (dormant or discontinued) projects.
* Recurrent Glioblastoma Drugs under development based on the stage of development, route of administration, target receptor, monotherapy or combination therapy, a different mechanism of action, and molecular type.
* Detailed analysis of collaborations (company-company collaborations and company-academia collaborations), licensing agreement and financing details for future advancement of the Recurrent Glioblastoma Market
Stay informed about the Recurrent Glioblastoma Pipeline trends! Uncover critical updates on therapeutic innovations and their potential impact on patients and the healthcare industry @ Recurrent Glioblastoma Unmet Needs [https://www.delveinsight.com/sample-request/recurrent-glioblastoma-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Recurrent Glioblastoma Companies
Ascletis, Genexine, PharmAbcine, VAXIMM AG, WPD Pharmaceuticals, Accendatech USA Inc., Midatech Ltd, MediciNova, Kadmon Corporation, LLC, Istari Oncology, Inc., Bristol-Myers Squibb, Novartis, Jiangsu Hengrui Medicine, Peloton Therapeutics, Inc., Karyopharm Therapeutics, VBL Therapeutics, Nerviano Medical Sciences, Acerta Pharma BV, Basilea Pharmaceutica, DNAtrix, Inc., NanoPharmaceuticals LLC, Erasca, Inc., Oblato, Inc., OX2 Therapeutics, Crimson Biopharm Inc., Merck Sharp & Dohme LLC, Transgene, CANbridge Life Sciences Ltd., Eli Lilly and Company, Arcus Biosciences, Inc., Incyte Corporation, BerGenBio ASA, Istari Oncology, Inc., and Chimerix and others.
Recurrent Glioblastoma pipeline report provides the therapeutic assessment of the pipeline drugs by the Route of Administration. Products have been categorized under various ROAs such as
* Inhalation
* Inhalation/Intravenous/Oral
* Intranasal
* Intravenous
* Intravenous/ Subcutaneous
* NA
* Oral
* Oral/intranasal/subcutaneous
* Parenteral
* Subcutaneous
Recurrent Glioblastoma Products have been categorized under various Molecule types such as
* Antibody
* Antisense oligonucleotides
* Immunotherapy
* Monoclonal antibody
* Peptides
* Protein
* Recombinant protein
* Small molecule
* Stem Cell
* Vaccine
Transform your understanding of the Recurrent Glioblastoma Pipeline! See the latest progress in drug development and clinical research @ Recurrent Glioblastoma Market Drivers and Barriers, and Future Perspectives [https://www.delveinsight.com/sample-request/recurrent-glioblastoma-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Scope of the Recurrent Glioblastoma Pipeline Report
* Coverage- Global
* Recurrent Glioblastoma Companies- Ascletis, Genexine, PharmAbcine, VAXIMM AG, WPD Pharmaceuticals, Accendatech USA Inc., Midatech Ltd, MediciNova, Kadmon Corporation, LLC, Istari Oncology, Inc., Bristol-Myers Squibb, Novartis, Jiangsu Hengrui Medicine, Peloton Therapeutics, Inc., Karyopharm Therapeutics, VBL Therapeutics, Nerviano Medical Sciences, Acerta Pharma BV, Basilea Pharmaceutica, DNAtrix, Inc., NanoPharmaceuticals LLC, Erasca, Inc., Oblato, Inc., OX2 Therapeutics, Crimson Biopharm Inc., Merck Sharp & Dohme LLC, Transgene, CANbridge Life Sciences Ltd., Eli Lilly and Company, Arcus Biosciences, Inc., Incyte Corporation, BerGenBio ASA, Istari Oncology, Inc., and Chimerix and others.
* Recurrent Glioblastoma Pipeline Therapies- Pembrolizumab, Olaparib, Temozolomide, BIBF1120, Chemotherapy, GX-I7, Bevacizumab, Bevacizumab, TTAC-0001, Cediranib and others.
* Recurrent Glioblastoma Therapeutic Assessment by Product Type: Mono, Combination, Mono/Combination
* Recurrent Glioblastoma Therapeutic Assessment by Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
Stay Ahead in Oncology Research-Access the Full Recurrent Glioblastoma Pipeline Analysis Today! @ Recurrent Glioblastoma Drugs and Companies [https://www.delveinsight.com/sample-request/recurrent-glioblastoma-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Table of Contents
* Introduction
* Executive Summary
* Recurrent Glioblastoma: Overview
* Pipeline Therapeutics
* Therapeutic Assessment
* Recurrent Glioblastoma - DelveInsight's Analytical Perspective
* Late Stage Products (Phase III)
* ASC40: Ascletis
* Drug profiles in the detailed report.....
* Mid Stage Products (Phase II)
* GX-I7: Genexine
* Drug profiles in the detailed report.....
* Early Stage Products (Phase I/II)
* VXM01: VAXIMM AG
* Drug profiles in the detailed report.....
* Preclinical and Discovery Stage Products
* Drug name: Company name
* Drug profiles in the detailed report.....
* Inactive Products
* Recurrent Glioblastoma Key Companies
* Recurrent Glioblastoma Key Products
* Recurrent Glioblastoma- Unmet Needs
* Recurrent Glioblastoma- Market Drivers and Barriers
* Recurrent Glioblastoma- Future Perspectives and Conclusion
* Recurrent Glioblastoma Analyst Views
* Recurrent Glioblastoma Key Companies
* Appendix
About Us
DelveInsight is a leading healthcare-focused market research and consulting firm that provides clients with high-quality market intelligence and analysis to support informed business decisions. With a team of experienced industry experts and a deep understanding of the life sciences and healthcare sectors, we offer customized research solutions and insights to clients across the globe. Connect with us to get high-quality, accurate, and real-time intelligence to stay ahead of the growth curve.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Yash Bhardwaj
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=recurrent-glioblastoma-pipeline-appears-promising-with-50-leading-biotech-and-pharma-companies-driving-innovation-in-the-therapeutics-segment-delveinsight]
Phone: 09650213330
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: NV
Country: United States
Website: https://www.delveinsight.com/report-store/recurrent-glioblastoma-pipeline-insight
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Recurrent Glioblastoma Pipeline Appears Promising With 50+ Leading Biotech and Pharma Companies Driving Innovation in the Therapeutics Segment | DelveInsight here
News-ID: 4108811 • Views: …
More Releases from ABNewswire

New $29 Guide Teaches First-Time Homebuyers How to Purchase a Home Without a Ban …
With mortgage rates above 6% and often higher for buyers with limited credit, the NoBankBuy guide helps everyday Americans legally take over existing low-interest mortgages through subject-to real estate financing - no credit check and no bank approval required.
LARGO, FL - February 18, 2026 - With mortgage rates above 6% and strict lending requirements disqualifying millions of potential buyers, homeownership remains out of reach for many working Americans. NoBankBuy (nobankbuy.com),…

High-Protein Meals for Fitness Enthusiasts Across Vancouver
2 Guys With Knives delivers high-protein meals designed specifically for fitness enthusiasts and athletes across Metro Vancouver, providing 150+ grams of protein daily through chef-prepared, ready to eat meals that support muscle growth, recovery, and athletic performance.
VANCOUVER, BC - 2 Guys With Knives announces the continued success of its high-protein meal offerings, designed specifically for fitness enthusiasts, athletes, and individuals focused on muscle development and recovery. The Vancouver-based meal prep…
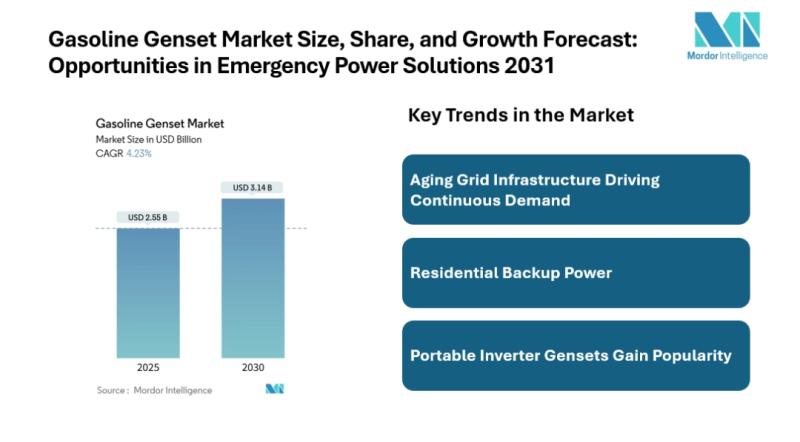
Gasoline Genset Market Size to rise to $ 3.14 Billion by 2030 | Standby and Port …
Mordor Intelligence has published a new report on the Gasoline Genset Market, offering a comprehensive analysis of trends, growth drivers, and future projections.
The global gasoline genset market size estimated at USD 2.55 billion in 2025 and projected to reach USD 3.14 billion by 2030. This represents a CAGR of 4.23% 2025-2030.
The demand is anchored by ongoing vulnerabilities in power grids, increasing urban construction activity in emerging economies, and a growing…

Tofu Market Size to Reach USD 3.77 Billion by 2031 as Flexitarian Diets and Prod …
Mordor Intelligence has published a comprehensive analysis of the tofu market, highlighting long-term growth drivers, evolving consumer trends, and competitive positioning through 2031.
Tofu Market Forecast, Size, and Growth Outlook
According to a research report by Mordor Intelligence, the global tofu market size [https://www.mordorintelligence.com/industry-reports/tofu-market?utm_source=abnewswire] is projected to grow from USD 2.08 billion in 2026 to USD 3.77 billion by 2031, reflecting strong tofu market growth during the forecast period. This expansion is…
More Releases for Recurrent
Recurrent Glioblastoma Pipeline Outlook Report 2024 (Updated)
DelveInsight's, "Recurrent Glioblastoma Pipeline Insight 2024" report provides comprehensive insights about 50+ companies and 50+ pipeline drugs in Recurrent Glioblastoma pipeline landscape. It covers the pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Key Takeaways from the Recurrent Glioblastoma Pipeline Report
DelveInsight's Recurrent Glioblastoma…
Recurrent Glioblastoma Pipeline, FDA Approvals, Clinical Trials Developments and …
DelveInsight's, "Recurrent Glioblastoma Pipeline Insight 2024" report provides comprehensive insights about 50+ Recurrent Glioblastoma companies and 50+ pipeline drugs in Recurrent Glioblastoma pipeline landscape. It covers the Recurrent Glioblastoma pipeline drug profiles, including clinical and nonclinical stage products. It also covers the Recurrent Glioblastoma pipeline therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Key Takeaways from…
Recurrent Glioblastoma Pipeline, FDA Approvals, Clinical Trials Assessment and C …
DelveInsight's, "Recurrent Glioblastoma Pipeline Insight" report provides comprehensive insights about 50+ Recurrent Glioblastoma companies and 50+ pipeline drugs in Recurrent Glioblastoma pipeline landscape. It covers the Recurrent Glioblastoma pipeline drug profiles, including clinical and nonclinical stage products. It also covers the Recurrent Glioblastoma pipeline therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Key Takeaways from the…
Recurrent Vulvovaginal Candidiasis Market - Defeating Recurrent Vulvovaginal Can …
Newark, New Castle, USA: The "Recurrent Vulvovaginal Candidiasis Market" provides a value chain analysis of revenue for the anticipated period from 2023 to 2031. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors.
Recurrent Vulvovaginal Candidiasis Market: https://www.growthplusreports.com/report/recurrent-vulvovaginal-candidiasis-market/8832
This latest report researches the industry structure,…
Recurrent Malignant Glioma Pipeline Therapeutics Development Market Report in H1 …
ReportsnReports.com added Recurrent Malignant Glioma - Pipeline is new Oncology report. The pipeline guide reviews latest news related to pipeline therapeutics for Recurrent Malignant Glioma (Oncology).
Complete report @ http://www.reportsnreports.com/reports/1117831-recurrent-malignant-glioma-pipeline-review-h1-2017.html
Key players of Recurrent Malignant Glioma Pipeline – AbbVie Inc and GtreeBNT Co Ltd
Recurrent Malignant Glioma - Drug Profiles - Cellular Immunotherapy for Recurrent Malignant Glioma, Cellular Immunotherapy to Target EphA2 for Recurrent Malignant Glioma, Cellular…
Global Recurrent Glioblastoma Multiforme (GBM) Treatment Market, 2016 - 2022
Glioblastoma multiforme (GBM) is the most common and aggressive malignant brain tumor in humans that involves glial cells. GBM is present in two variants namely, giant cell glioblastoma and gliosarcoma. Gliomas are tumors arising from glial cells and may occur in the spinal cord or the brain, the latter being more common. Gliomas are the most common type of brain tumor and can be either supratentorial or infratentorial. Seizure,…
