Press release
GMP Cell Therapy Consumables Market Trends That Will Shape the Next Decade: Insights from Advancements in GMP-Compliant CD34+ Hematopoietic Stem Cells: OrganaBio's Launch and Industry Developments
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.How Large Will the GMP Cell Therapy Consumables Market Size By 2025?
In recent times, the market size of GMP cell therapy consumables has seen rapid expansion. The market is expected to rise from $17.85 billion in 2024 and reach a sizing of $22.87 billion in 2025, representing a compound annual growth rate (CAGR) of 28.1%. Factors such as increased investment in regenerative medicine, improved cell therapy research, clinical trials and approvals, the mounting incidence of chronic illnesses, and the heightened understanding of personalized medicine have all contributed to the growth observed in the historical period.
How Big Is the GMP Cell Therapy Consumables Market Size Expected to Grow by 2029?
The market size of GMP cell therapy consumables is anticipated to witness a tremendous increase in the coming years. It is predicted to reach $59 billion in 2029 with a CAGR of 26.7%. The surge during the forecasted period is linked to the expansion of cell therapy applications, a worldwide increase in cell therapy trials, regulatory backing for cell therapies, the introduction of sophisticated cell therapy platforms, and the globalization of cell therapy manufacturing. Key trends projected in the forecast period encompass investment in manufacturing infrastructure, technological innovation in cell processing, collaborative initiatives and partnerships, digitization and data integration, along with strategic alliances and mergers.
View the full report here:
https://www.thebusinessresearchcompany.com/report/gmp-cell-therapy-consumables-global-market-report
Which Key Market Drivers Powering GMP Cell Therapy Consumables Market Expansion and Growth?
The upward trend in drug discovery is projected to be a key driver for the expansion of the GMP cell therapy consumables market. Drug discovery encompasses finding new potential medicinal substances by merging computational, clinical, experimental, and translational models. Breakthroughs in bioinformatics, genetics, molecular biology, and high-throughput screening techniques have transformed the realm of drug discovery. These cutting-edge technologies empower researchers to better comprehend disease mechanisms, pinpoint drug targets, and screen vast compound libraries for potent drug contenders more proficiently. This surge in drug discovery is boosting the GMP cell therapy consumables market. For example, in January 2023, the Food and Drug Administration - a federal agency under the US Department of Health and Human Services, reported that the FDA's Centre for Drug Evaluation and Research (CDER) approved 37 new drugs and therapeutic biological products in the US in 2022 for various medical conditions. As a result, the surge in drug discovery is fueling the expansion of the GMP cell therapy consumables market.
Get your free sample here:
https://www.thebusinessresearchcompany.com/sample.aspx?id=12014&type=smp
Which Fast-Growing Trends Are Poised to Disrupt the GMP Cell Therapy Consumables Market?
Leading firms in the GMP cell therapy consumables market are producing GMP-compliant CD34+ hematopoietic stem cells (HSCs) to assist advanced therapies. GMP-compliant CD34+ HSCs are a unique type of stem cell that exhibit the CD34 marker on their surface and are produced corresponding to Good Manufacturing Practice (GMP) standards. For example, in April 2024, the US-based pharmaceutical firm OrganaBio presented HematoPAC-HSC-CB-GMP, a readily accessible source of GMP-compliant CD34+ HSCs obtained from fresh human umbilical cord blood. OrganaBio calls upon its vast experience in cell isolation and GMP production to generate abundant amounts of highly viable CD34+ HSCs within a day of collection. The introduction of this product is geared towards fostering the advancement of next-generation treatments, including therapies for blood cancers and genetic disorders.
What Are the Emerging Segments in the GMP Cell Therapy Consumables Market?
The GMP cell therapy consumables market covered in this report is segmented -
1) By Product: Kits, Reagents Or Molecular Biology reagents, Growth Factors Or Cytokines And Interleukins, Other Products
2) By Cell Therapy: NK Cell Therapy, Stem Cell Therapy, T-Cell Therapy, Other cell therapies
3) By Process: Cell Collection And Characterization Or Sorting And Separation, Cell Culture And Expansion Or Preparation, Cryopreservation, Cell Processing And Formulation, Cell Isolation And Activation, Cell Distribution Or Handling, Process Monitoring And Control Or Readministration Or Quality Assurance, Other Processes
4) By End-Use: Clinical, Commercial, Research
Subsegments:
1) By Kits: Cell Culture Kits, Cell Isolation Kits, Transfection Kits
2) By Reagents Or Molecular Biology Reagents: Buffers And Solutions, Enzymes, Staining Reagents
3) By Growth Factors Or Cytokines And Interleukins: Hematopoietic Growth Factors, Antibody Cytokines, Immunomodulatory Cytokines
3) By Other Products: Cell Culture Vessels, Bioreactors, Media And Supplements
Tailor your insights and customize the full report here:
https://www.thebusinessresearchcompany.com/customise?id=12014&type=smp
Who Are the Global Leaders in the GMP Cell Therapy Consumables Market?
Major companies operating in the GMP cell therapy consumables market include Thermo Fisher Scientific Inc., Fresenius Kabi AG, Danaher Corporation, Merck KGaA, Asahi Kasei Corporation, GE HealthCare Technologies Inc., Corning Incorporated, Avantor Inc., Lonza Group AG, Terumo Corporation, Catalent Inc., Sartorius AG, Bio-Techne Corp, Repligen Corporation, Miltenyi Biotec BV & Co KG, Genscript Biotech Corporation, Rentschler Biopharma SE, FUJIFILM Irvine Scientific Inc., BioLegend Inc., STEMCELL Technologies Inc., BioLife Solutions Inc., Abzena Ltd., Sino Biological Inc., ProBioGen AG, MaxCyte Inc., PromoCell GmbH, PeproTech Inc., Cellares Corp., Wilson Wolf Corporation, Cellexus Ltd.
Which are the Top Profitable Regional Markets for the GMP Cell Therapy Consumables Industry?
North America was the largest region in the GMP cell therapy consumables market in 2024. Asia-pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the GMP cell therapy consumables market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa.
Purchase the full report today:
https://www.thebusinessresearchcompany.com/purchaseoptions.aspx?id=12014
This Report Supports:
1. Business Leaders & Investors - To identify growth opportunities, assess risks, and guide strategic decisions.
2. Manufacturers & Suppliers - To understand market trends, customer demand, and competitive positioning.
3. Policy Makers & Regulators - To track industry developments and align regulatory frameworks.
4. Consultants & Analysts - To support market entry, expansion strategies, and client advisory work.
Connect with us on:
LinkedIn: https://in.linkedin.com/company/the-business-research-company,
Twitter: https://twitter.com/tbrc_info,
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ.
Contact Us
Europe: +44 7882 955267,
Asia: +91 88972 63534,
Americas: +1 310-496-7795 or
Email: mailto:info@tbrc.info
Learn More About The Business Research Company
With over 15,000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Our flagship product, the Global Market Model delivers comprehensive and updated forecasts to support informed decision-making.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release GMP Cell Therapy Consumables Market Trends That Will Shape the Next Decade: Insights from Advancements in GMP-Compliant CD34+ Hematopoietic Stem Cells: OrganaBio's Launch and Industry Developments here
News-ID: 4105650 • Views: …
More Releases from The Business Research Company
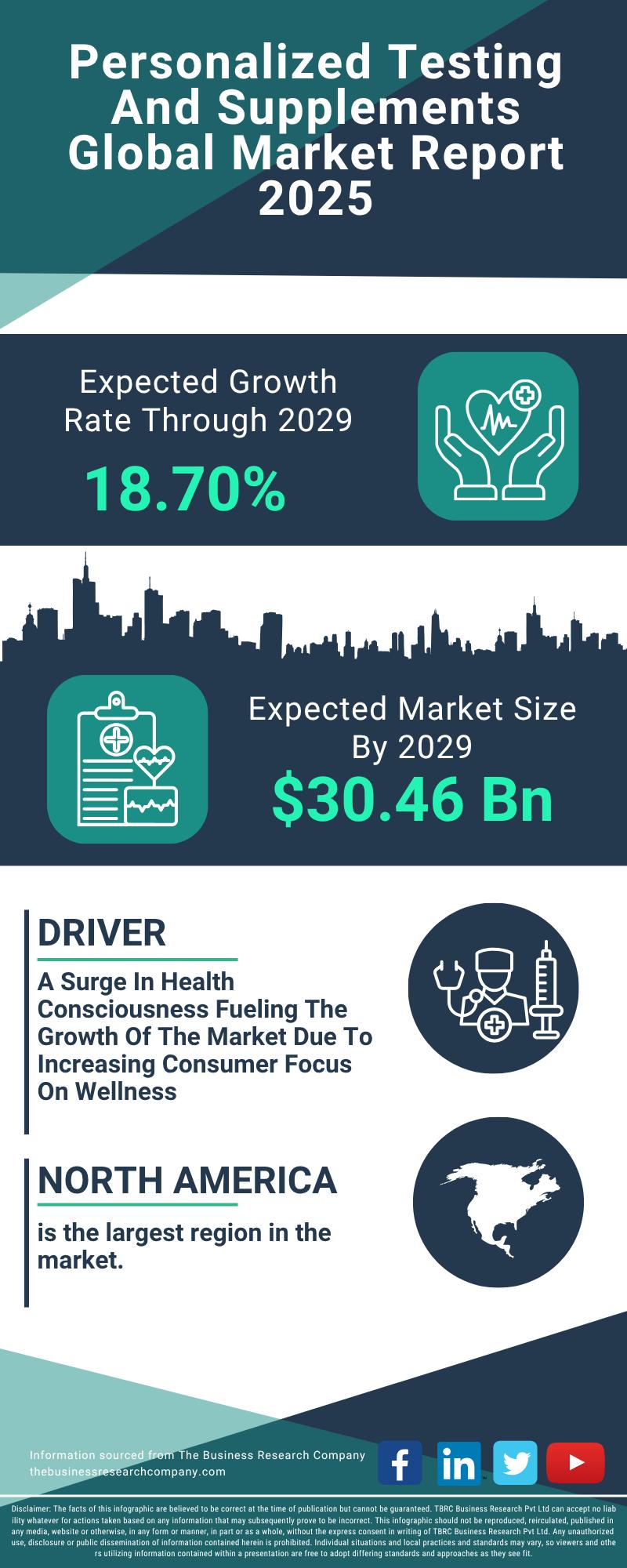
Segment Evaluation and Major Growth Areas in the Personalized Testing and Supple …
The personalized testing and supplements sector is gaining remarkable traction, driven by advancements in technology and a rising consumer focus on tailored health solutions. As more individuals seek customized wellness options, this market is set to experience substantial expansion in the coming years. Here's an in-depth look at its current valuation, key players, significant trends, and the main market segments shaping its future.
Market Valuation and Expansion Forecast for Personalized Testing…

Top Players and Market Competition in the Skin Microbiome Industry
The skin microbiome market is emerging as a significant area of interest due to growing awareness about the critical role of skin health and innovative skincare technologies. As research advances and consumer preferences shift towards more natural and science-backed products, this market is set to undergo substantial growth. Let's explore the current market size, key players, driving factors, and upcoming trends shaping the skin microbiome industry.
Projected Expansion in the Skin…
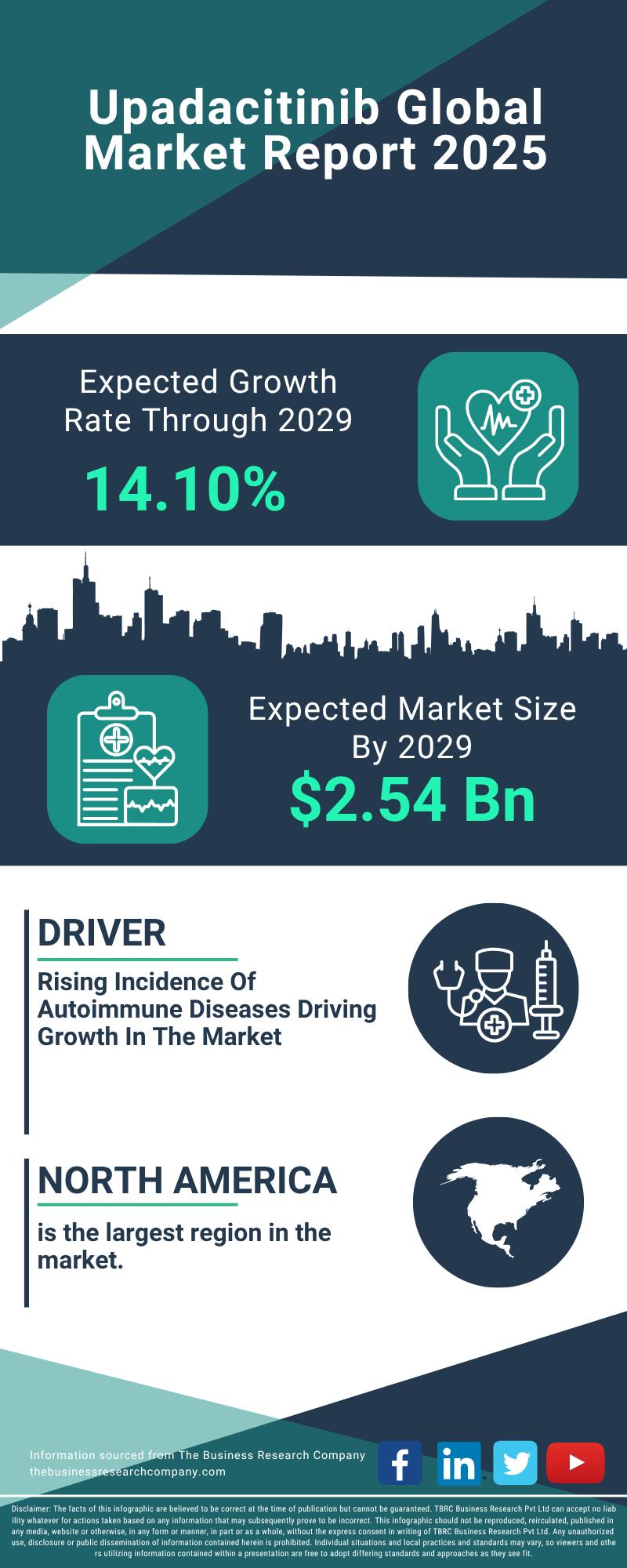
Key Strategic Developments and Emerging Changes Shaping the Upadacitinib Market …
The upadacitinib market is poised for significant expansion over the coming years, driven by advances in treatment options and increasing awareness of autoimmune diseases. This report delves into the market's current size, key drivers, major players, and the emerging trends shaping its future trajectory.
Steady Growth Expected in Upadacitinib Market Size Through 2029
The market for upadacitinib is projected to reach $2.54 billion by 2029, growing at a robust compound annual…

Analysis of Key Market Segments Driving the Alzheimer's Disease Diagnostic Marke …
The Alzheimer's disease diagnostic sector is rapidly evolving as advancements in technology and healthcare infrastructure open new possibilities for early detection and personalized treatment. With rising awareness and innovative approaches, this market is poised for significant growth in the coming years. Let's explore the current market size, key drivers, leading companies, and emerging trends that are shaping this critical healthcare field.
Projected Market Size and Growth Trends in Alzheimer's Disease Diagnostics…
More Releases for GMP
Creative Peptides Released GMP Synthesis Service
Located in Shirley, New York, the world’s leading peptide supplier Creative Peptides announced the launch of its GMP synthesis (https://www.creative-peptides.com/services/custom-gmp-peptide-synthesis-services.html ) business on August 29, 2018. Now this company is focused on the development and GMP manufacturing of pharmaceutical grade peptides.
As the demand of pharmaceutical market continues to grow, more and more pharmas and research institutions choose the CMO and CRO models to expand their businesses, which is more…
Diapharm implements European GMP guidelines in China
Münster (DE), London (UK), Ningbo (CN), 20 December 2013 – Pharmaceutical service provider Diapharm (diapharm.com) is increasing its business activities in China: Diapharm has now implemented a “European” quality management system for Neptune Pharma Ltd (www.neptunepharma.com) in their Joint Venture Partner’s factory in Ningbo, Zhejiang Province. And it has done so successfully: The veterinary medicinal product Trident 500mg/g Powder for Suspension for Fish Treatment (www.trident-50.com), is manufactured onsite under EU…
ECA Foundation releases free GMP WebApp
The ECA Foundation has been providing advanced training and information services in the pharmaceutical industry and especially with regard to pharmaceutical Quality Assurance and GMP compliance for more than 10 years. Now the organisation took advantage of its extensive experience to develop a further free of charge service – the new GMP WebApp.
This new GMP WebApp runs on all smartphones and tablet PCs (Apple and Android platforms) and allows users…
GMP Friction Products Awarded ISO 9001:2008
Internationally Recognized Certification Measures Consistency in Process, Procedure and Quality Performance in Manufacture of Friction Materials
AKRON, OH (March 23, 2011) -- GMP Friction Products, a world leader manufacturing powdered metal friction products for clutch plates and brake pads, recently received certification for ISO 9001:2008.
“ISO 9001:2008 signifies we have taken the extra measure of documenting the policies and standards to ensure consistent compliance with our manufacturing processes,” said Jerry Lynch,…
GMP MANUAL Volume 2 - Validation Procedures by Maas & Peither AG – GMP Publish …
GMP Publishing is launching its new GMP MANUAL Volume 2 – Validation Procedures.
The compendium on validation procedures was written by Dr. Doris Borchert, Dr. Peter Bosshard, Dr. Ralph Gomez, Dr. Michael Hiob, Dr. Christine Oechslein, Max Lazar, Ulrike Reuter, Michael Schulte, Uwe Schwarzat – all international experts and key opinion leaders. They share their detailed understanding of the various aspects of the validation process in clear and comprehensive style…
blue inspection body celebrates 50 GMP audits
Münster (Germany), 20 November 2009. Two years after founding the company and just 18 months after gaining the accreditation blue inspection body GmbH announced today the successful execution of its 50th GMP audit. Further audit trips to China, India, Israel and various European countries have been scheduled already, meaning that in the first quarter 2010 the 75th audit is targeted to be completed. Blue, as a privately organised inspection body,…
