Press release
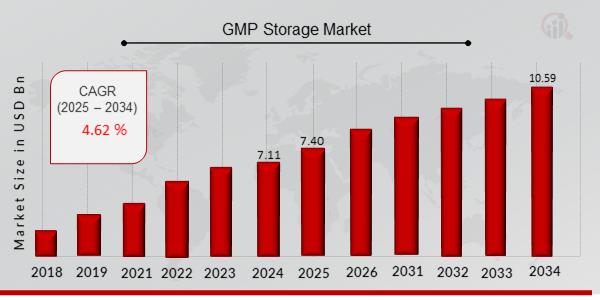
GMP Storage Market Projected to Hit USD 10.59 Billion by 2034, at a Exceptional CAGR 4.06%
Optimizing GMP Storage: A Critical Element in Quality ComplianceGood Manufacturing Practices (GMP) are essential for industries like pharmaceuticals, biotechnology, food, and cosmetics, where product safety, efficacy, and quality are paramount. One of the most overlooked yet critical components of GMP is proper storage. GMP storage refers to the structured and compliant way of storing raw materials, intermediates, and finished products to ensure they maintain their intended quality throughout their lifecycle.
The GMP Storage Market was valued at USD 7.11 billion in 2024 and is poised for steady growth in the coming years. With an estimated value of USD 7.40 billion in 2025, the market is projected to reach approximately USD 10.59 billion by 2034, expanding at a compound annual growth rate (CAGR) of 4.06% during the forecast period from 2025 to 2034. This growth is primarily driven by the increasing demand for compliant and secure storage solutions in the pharmaceutical and biotechnology sectors, where maintaining product integrity and regulatory standards is critical. The market is also benefiting from rising investments in life sciences infrastructure, growing biopharmaceutical production, and advancements in temperature-controlled storage technologies.
Request To Free Sample of This Strategic Report: https://www.marketresearchfuture.com/sample_request/37296
Key Companies in the GMP Storage Market Include:
Danaher Corporation
Avantor
GE Healthcare
Sartorius AG
Berhorn GmbH
VWR International
Thermo Fisher Scientific
Agilent Technologies
Hirschmann Laborgeräte GmbH
Corning Incorporated
Waters Corporation
Merck KGaA
Fisher Scientific
PerkinElmer
Eppendorf AG
The Importance of GMP-Compliant Storage
Storage is not merely about shelving products; it plays a direct role in maintaining product integrity. Inadequate storage conditions can lead to contamination, degradation, or even complete product failure, risking consumer safety and leading to costly product recalls or regulatory action. GMP guidelines ensure that every element of storage - from the temperature and humidity to cleanliness and traceability - is carefully controlled and documented.
You Can Purchase Complete Report: https://www.marketresearchfuture.com/checkout?currency=one_user-USD&report_id=37296
GMP Storage Market Segmentation Insights
GMP Storage Market Application Outlook
Pharmaceuticals
Biotechnology
Clinical Research
GMP Storage Market Storage Type Outlook
Cold Storage
Controlled Room Temperature Storage
Ambient Storage
GMP Storage Market End User Outlook
Pharmaceutical Companies
Contract Research Organizations
Research Institutions
GMP Storage Market Component Outlook
Storage Equipment
Monitoring Systems
Accessories
GMP Storage Market Regional Outlook
North America
Europe
South America
Asia Pacific
Middle East and Africa
Browse In-depth Market Research Report (Pages, Charts, Tables, Figures): https://www.marketresearchfuture.com/reports/gmp-storage-market-37296
Key Principles of GMP Storage
1. Environmental Control:
Temperature, humidity, and light exposure must be monitored and regulated. For instance, sensitive APIs (Active Pharmaceutical Ingredients) and vaccines may require refrigeration or cold chain storage. Modern GMP storage facilities are equipped with automated monitoring systems and alarms to prevent excursions beyond allowable ranges.
2. Segregation and Labeling:
GMP standards call for strict segregation of materials based on their type and status - raw materials, intermediates, quarantined goods, rejected items, and released products must all be stored in clearly marked areas. Proper labeling ensures traceability and avoids cross-contamination or misuse.
3. Cleanliness and Sanitation:
Storage areas must be clean, pest-free, and maintained under a regular cleaning schedule. Floors, walls, and storage racks should be made of non-porous materials that are easy to clean. Sanitation logs and cleaning protocols must be diligently maintained to comply with inspection standards.
4. Documentation and Traceability:
Every movement and status change of materials must be documented. GMP storage involves robust inventory management systems that provide real-time data on stock levels, expiration dates, batch numbers, and storage conditions. This traceability helps in quality control and quick action during investigations or recalls.
5. Security and Access Control:
Only authorized personnel should have access to GMP storage areas. Access control measures - such as RFID badges, biometric scanners, and audit trails - are essential for maintaining the integrity of stored products.
Emerging Trends in GMP Storage
With the increasing complexity of supply chains and the globalization of manufacturing, the role of GMP-compliant storage is evolving. Automation and IoT-based monitoring systems are becoming common, offering real-time alerts and predictive maintenance capabilities. Furthermore, the adoption of eco-friendly materials and energy-efficient refrigeration units aligns with both regulatory compliance and sustainability goals.
Related Report:
Uk Contract Research Organization Market : https://www.marketresearchfuture.com/reports/uk-contract-research-organization-market-44077
China Medical Tourism Market : https://www.marketresearchfuture.com/reports/china-medical-tourism-market-44099
India Medical Tourism Market : https://www.marketresearchfuture.com/reports/india-medical-tourism-market-44098
Japan Medical Tourism Market : https://www.marketresearchfuture.com/reports/japan-medical-tourism-market-44093
South America Medical Tourism Market : https://www.marketresearchfuture.com/reports/south-america-medical-tourism-market-44097
South Korea Medical Tourism Market : https://www.marketresearchfuture.com/reports/south-korea-medical-tourism-market-44092
South Korea Telehealth Market : https://www.marketresearchfuture.com/reports/south-korea-telehealth-market-44088
Uk Telehealth Market : https://www.marketresearchfuture.com/reports/uk-telehealth-market-44087
Us Telehealth Market : https://www.marketresearchfuture.com/reports/us-telehealth-market-44089
Germany Ehr Emr Market : https://www.marketresearchfuture.com/reports/germany-ehr-emr-market-44687
Contact:
Market Research Future (Part of Wantstats Research and Media Private Limited)
99 Hudson Street, 5Th Floor
New York, NY 10013
United States of America
+1 628 258 0071 (US)
+44 2035 002 764 (UK)
Email: sales@marketresearchfuture.com
Website: https://www.marketresearchfuture.com
About Market Research Future:
Market Research Future (MRFR) is a global market research company that takes pride in its services, offering a complete and accurate analysis with regard to diverse markets and consumers worldwide. Market Research Future has the distinguished objective of providing the optimal quality research and granular research to clients. Our market research studies by products, services, technologies, applications, end users, and market players for global, regional, and country level market segments, enable our clients to see more, know more, and do more, which help answer your most important questions.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release GMP Storage Market Projected to Hit USD 10.59 Billion by 2034, at a Exceptional CAGR 4.06% here
News-ID: 4104437 • Views: …
More Releases from Market Research Future

3D Printing Filament Industry Projected at USD 26.78 Billion by 2035 with 24.52% …
The global 3D Printing Filament Market is experiencing an unprecedented boom, transitioning rapidly from a niche prototyping tool to a cornerstone of modern, decentralized manufacturing. As additive manufacturing permeates industries from aerospace to education, and even reaches hobbyists' homes, the demand for the specialized materials that bring digital designs to life-the filaments-is skyrocketing. According to a comprehensive report by Market Research Future, the market is projected to explode from an…

Proppants Market to Reach USD 29.87 Billion by 2035 from USD 10.31 Billion in 20 …
The global Proppants Market is poised for explosive growth, driven by the relentless global demand for energy and the technological mastery of unconventional resource extraction. As the "pickaxe" of the 21st-century oilfield, proppants-small, durable particles used to prop open fractures in rock formations-are essential for unlocking oil and gas from shale and other tight reservoirs. According to a comprehensive report by Market Research Future, the market is projected to skyrocket…

Infection Control Market Forecasted to Reach USD 81.05 Billion By 2035, at an Im …
Enhancing Safety and Care: The Growing Importance of Infection Control
In today's healthcare environment, infection control has emerged as a cornerstone of patient safety and overall public health. Hospitals, clinics, and laboratories worldwide are increasingly recognizing that rigorous infection control measures are not just regulatory requirements-they are essential practices that protect patients, healthcare workers, and the broader community. From the prevention of hospital-acquired infections to combating global outbreaks, effective infection control…
Hematology Diagnostics Market Expected to Achieve a Strong 3.2% CAGR, to Reach U …
Advancing Patient Care Through Hematology Diagnostics
Hematology diagnostics play a pivotal role in modern healthcare by providing critical insights into blood-related disorders and overall patient health. From routine blood counts to advanced molecular testing, hematology diagnostics enable clinicians to detect, monitor, and manage conditions such as anemia, leukemia, clotting disorders, and other hematologic diseases. The continuous evolution in diagnostic technologies is improving accuracy, reducing turnaround times, and empowering physicians to make…
More Releases for GMP
Creative Peptides Released GMP Synthesis Service
Located in Shirley, New York, the world’s leading peptide supplier Creative Peptides announced the launch of its GMP synthesis (https://www.creative-peptides.com/services/custom-gmp-peptide-synthesis-services.html ) business on August 29, 2018. Now this company is focused on the development and GMP manufacturing of pharmaceutical grade peptides.
As the demand of pharmaceutical market continues to grow, more and more pharmas and research institutions choose the CMO and CRO models to expand their businesses, which is more…
Diapharm implements European GMP guidelines in China
Münster (DE), London (UK), Ningbo (CN), 20 December 2013 – Pharmaceutical service provider Diapharm (diapharm.com) is increasing its business activities in China: Diapharm has now implemented a “European” quality management system for Neptune Pharma Ltd (www.neptunepharma.com) in their Joint Venture Partner’s factory in Ningbo, Zhejiang Province. And it has done so successfully: The veterinary medicinal product Trident 500mg/g Powder for Suspension for Fish Treatment (www.trident-50.com), is manufactured onsite under EU…
ECA Foundation releases free GMP WebApp
The ECA Foundation has been providing advanced training and information services in the pharmaceutical industry and especially with regard to pharmaceutical Quality Assurance and GMP compliance for more than 10 years. Now the organisation took advantage of its extensive experience to develop a further free of charge service – the new GMP WebApp.
This new GMP WebApp runs on all smartphones and tablet PCs (Apple and Android platforms) and allows users…
GMP Friction Products Awarded ISO 9001:2008
Internationally Recognized Certification Measures Consistency in Process, Procedure and Quality Performance in Manufacture of Friction Materials
AKRON, OH (March 23, 2011) -- GMP Friction Products, a world leader manufacturing powdered metal friction products for clutch plates and brake pads, recently received certification for ISO 9001:2008.
“ISO 9001:2008 signifies we have taken the extra measure of documenting the policies and standards to ensure consistent compliance with our manufacturing processes,” said Jerry Lynch,…
GMP MANUAL Volume 2 - Validation Procedures by Maas & Peither AG – GMP Publish …
GMP Publishing is launching its new GMP MANUAL Volume 2 – Validation Procedures.
The compendium on validation procedures was written by Dr. Doris Borchert, Dr. Peter Bosshard, Dr. Ralph Gomez, Dr. Michael Hiob, Dr. Christine Oechslein, Max Lazar, Ulrike Reuter, Michael Schulte, Uwe Schwarzat – all international experts and key opinion leaders. They share their detailed understanding of the various aspects of the validation process in clear and comprehensive style…
blue inspection body celebrates 50 GMP audits
Münster (Germany), 20 November 2009. Two years after founding the company and just 18 months after gaining the accreditation blue inspection body GmbH announced today the successful execution of its 50th GMP audit. Further audit trips to China, India, Israel and various European countries have been scheduled already, meaning that in the first quarter 2010 the 75th audit is targeted to be completed. Blue, as a privately organised inspection body,…