Press release
Regulatory Affairs Outsourcing Market to Exceed US$ 22.3 Bn by 2035, Driven by Increasing Regulatory Complexity and Global Expansion Needs - Transparency Market Research
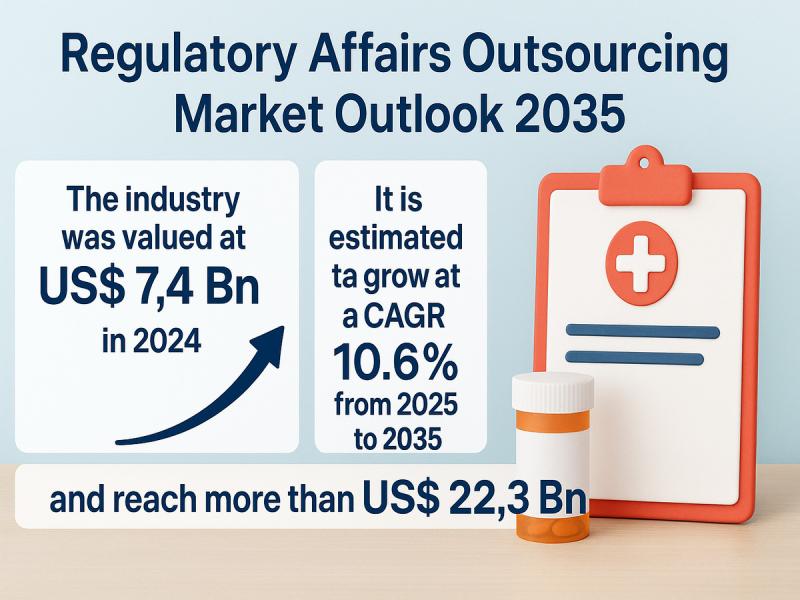
The global regulatory affairs outsourcing market was valued at US$ 7.4 Bn in 2024. It is projected to grow at a CAGR of 10.6% from 2025 to 2035, reaching over US$ 22.3 Bn by the end of 2035, according to a new report by Transparency Market Research. Heightened global regulatory scrutiny and rising demand for local representation across emerging markets are fueling outsourcing trends across pharmaceutical, biotechnology, and medical device industries.Get Sample PDF Brochure from here: https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=3528
Analysts' Viewpoint
The regulatory affairs outsourcing industry is transitioning from transactional support to a strategic partner role, as pharmaceutical and medtech firms face complex compliance demands. With regulatory requirements becoming more rigorous and region-specific, outsourcing partners offering end-to-end solutions-from clinical trial applications to post-market surveillance-are gaining traction. Demand is particularly high across Asia Pacific, Latin America, and the Middle East due to localization needs, expanding clinical trials, and emerging biotech hubs.
Overview: Navigating Complexity with External Expertise
Regulatory affairs outsourcing involves engaging third-party experts to ensure compliance across the entire product development and commercialization lifecycle. From regulatory writing and submissions to local representation and post-market lifecycle management, outsourcing helps firms stay ahead of changing global frameworks, reduce compliance risks, and optimize time-to-market.
The need for agile and expert-driven compliance support is particularly high among small- to mid-sized enterprises (SMEs) and multinational corporations seeking rapid global expansion. In this context, regulatory outsourcing has emerged as a strategic enabler of growth and operational resilience.
Key Market Drivers
Stringent Global Regulations Drive Outsourcing Demand
Accelerated innovation in medical devices and biologics has prompted tighter approval criteria and broader documentation requirements. Regulatory timelines are increasing due to complex evidence-based submissions, which can delay commercialization. Companies are mitigating these risks by outsourcing regulatory functions to specialized vendors that are continuously updated on evolving standards across the U.S. FDA, EMA, and other global agencies.
Notably, in November 2024, the Medical Device Coordination Group (MDCG) issued clarifications on Eudamed's rollout under the updated EU MDR and IVDR regulations. Such developments reinforce the need for expert-led submission strategies to prevent costly delays.
Local Representation Fuels Expansion into Emerging Markets
Global life science firms expanding into Asia Pacific, Latin America, and MEA face the challenge of country-specific protocols, language barriers, and diverse health authority expectations. Regulatory outsourcing enables access to in-country experts and on-ground infrastructure to handle applications, engage with authorities, and maintain compliance.
In March 2025, ICON plc expanded its regulatory services footprint in Asia Pacific, particularly in China and India, reflecting growing clinical activity and demand for localized regulatory support.
Segment Insights: Product Registration and Clinical Trial Applications Dominate
The product registration and clinical trial applications segment is anticipated to lead the market due to the globalization of trials, increased regulatory pressure, and legal obligations for representative presence. Growth is driven by both pre-market approvals and clinical documentation for fast-evolving therapeutic pipelines.
Complex regulatory scenarios in developing regions have heightened demand for legal representatives, making service providers with regional compliance infrastructure key growth facilitators.
Regional Outlook: North America Leads, Asia Pacific Emerging as a Growth Hub
North America
North America holds the dominant market share, estimated at 39.4% in 2024, supported by:
A large base of pharmaceutical and biotech companies
High number of clinical trials
Stringent oversight by agencies such as the FDA
Presence of CROs offering turnkey regulatory solutions
Asia Pacific
Asia Pacific is expected to be the fastest-growing region, led by rising pharma manufacturing capabilities, healthcare investments, and regulatory sophistication in China, India, and Japan. While cost-effectiveness remains a driver, improved service quality and local compliance maturity are enabling APAC to compete globally.
Competitive Landscape
The global regulatory affairs outsourcing market is moderately fragmented, with players offering tailored compliance strategies, tech-enabled workflows, and geographic coverage. Service providers are forming strategic partnerships with hospitals, labs, and trial centers to scale operations and enhance service scope.
Key Companies in the Regulatory Affairs Outsourcing Market
Accell Clinical Research, LLC
Genpact
CRITERIUM, INC
Promedica International
WuXi AppTec
Medpace
Charles River Laboratories
ICON plc
Labcorp Drug Development
Parexel International Corporation
Freyr
PHARMALEX GMBH
Recent Developments
August 2024 - LEAP Consulting Group introduced a consultancy model to help CLIA-certified laboratories align with expanded FDA LDT rules, reflecting broader oversight of laboratory-developed tests in the U.S.
October 2024 - ProductLife Group (PLG) acquired Callisto, a UK-based consultancy specializing in regulatory affairs, pharmacovigilance, and GMDP services, expanding its life sciences compliance portfolio.
Market Segmentation
By Service Type
Regulatory Consulting & Legal Representation
Product Registration & Clinical Trial Applications
Regulatory Writing & Publishing
Regulatory Submissions
Regulatory Operations
Others (Lifecycle/Post-approval Management)
By Product Development Stage
Preclinical
Clinical
PMA (Post-Market Authorization)
By Enterprise Size
Small/Medium Enterprises
Large Enterprises
By Therapeutic Area
Oncology
Neurology
Cardiology
Immunology
Dermatology
Others (e.g., Infectious Diseases)
By End-user
Medical Device Companies
Biopharma & Pharmaceutical Companies
Others (e.g., Biotech Companies)
By Region
North America
Europe
Asia Pacific
Latin America
Middle East & Africa
Buy this Premium Research Report:
https://www.transparencymarketresearch.com/checkout.php?rep_id=3528<ype=S
Explore Latest Research Reports by Transparency Market Research:
Clinical Laboratory Services Market- https://www.transparencymarketresearch.com/clinical-laboratory-services-market-report.html
U.S Ambulatory Infusion Centers Market- https://www.transparencymarketresearch.com/us-ambulatory-infusion-centers-market.html
Regulatory Affairs Outsourcing Market- https://www.transparencymarketresearch.com/global-regulatory-affairs-outsourcing-market.html
Corporate Wellness Market- https://www.transparencymarketresearch.com/corporate-wellness-market.html
Medical Tourism Market- https://www.transparencymarketresearch.com/medical-tourism-market.html
Contact Us:
Transparency Market Research Inc.
CORPORATE HEADQUARTER DOWNTOWN,
1000 N. West Street,
Suite 1200, Wilmington, Delaware 19801 USA
Tel: +1-518-618-1030
USA - Canada Toll Free: 866-552-3453
About Transparency Market Research
Transparency Market Research, a global market research company registered at Wilmington, Delaware, United States, provides custom research and consulting services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insights for thousands of decision makers. Our experienced team of Analysts, Researchers, and Consultants use proprietary data sources and various tools & techniques to gather and analyses information.
Our data repository is continuously updated and revised by a team of research experts, so that it always reflects the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in developing distinctive data sets and research material for business reports.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Regulatory Affairs Outsourcing Market to Exceed US$ 22.3 Bn by 2035, Driven by Increasing Regulatory Complexity and Global Expansion Needs - Transparency Market Research here
News-ID: 4098279 • Views: …
More Releases from Transparency Market Research
Electric Wheelchair Market Expanding at 9.2% CAGR Through 2036 - By Control Type …
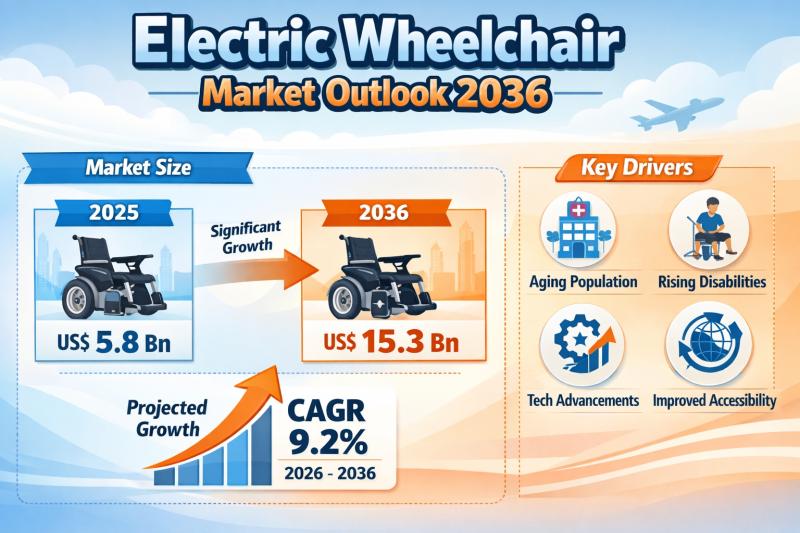
The global electric wheelchair market continues to demonstrate strong and sustained growth, fueled by demographic transitions, technological innovation, and expanding healthcare access worldwide. Valued at US$ 5.8 billion in 2025, the market is projected to reach US$ 15.3 billion by 2036, expanding at a compound annual growth rate (CAGR) of 9.2% from 2026 to 2036.
Discover essential conclusions and data from our Report in this sample -
https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=4198
This robust trajectory reflects rising…
Vehicle Predictive Maintenance Market Size Forecast to USD 13.7 Billion by 2036 …
Vehicle Predictive Maintenance Market Outlook 2036
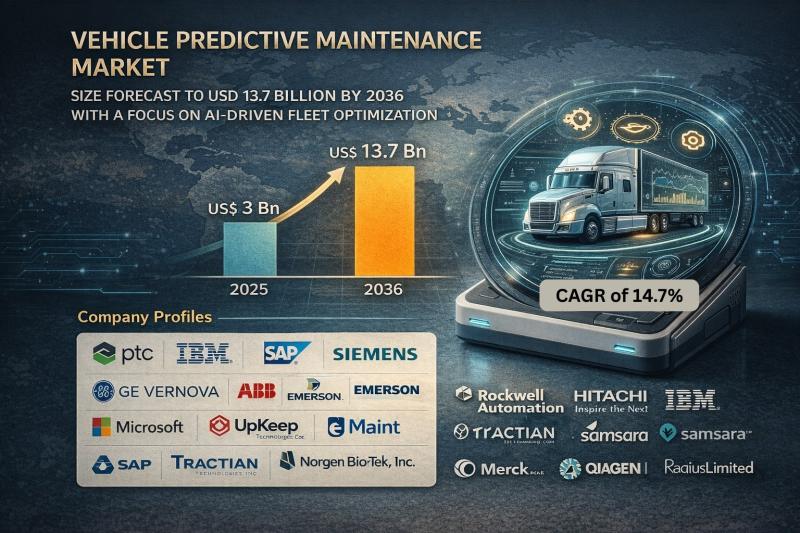
The global vehicle predictive maintenance market was valued at USD 3 Billion in 2025 and is projected to reach USD 13.7 Billion by 2036, expanding at a robust CAGR of 14.7% from 2026 to 2036. Market growth is driven by increasing adoption of connected vehicles, rising fleet digitalization, advancements in AI-driven analytics, and growing emphasis on minimizing vehicle downtime and maintenance costs.
👉 Get your sample…
Global Air Purification Systems Market to Reach USD 44.3 Billion by 2036 at 7.9% …
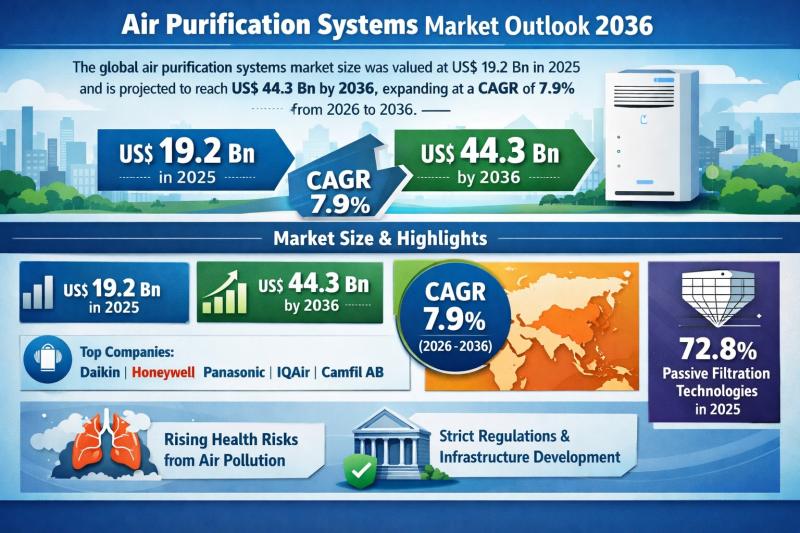
The global Air Purification Systems Market was valued at US$ 19.2 Bn in 2025 and is projected to expand to US$ 44.3 Bn by 2036, registering a compound annual growth rate (CAGR) of 7.9% from 2026 to 2036. The market's upward trajectory reflects the structural shift in indoor air quality (IAQ) management, moving from discretionary consumer spending to mission-critical infrastructure investment.
With historical data available from 2021 to 2024, the industry…
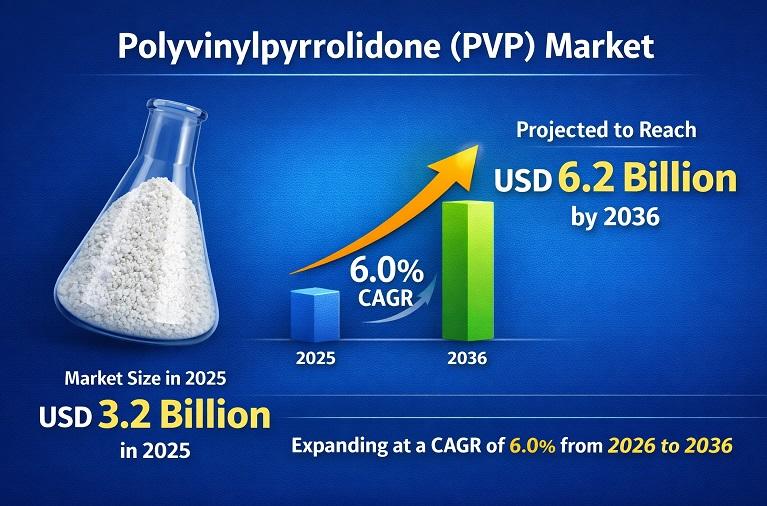
Polyvinylpyrrolidone (PVP) Market to Reach USD 6.2 Billion by 2036 Driven by Pha …
The Polyvinylpyrrolidone (PVP) Market was valued at around US$ 3.2 billion in 2025 and is projected to reach approximately US$ 6.2 billion by 2036, expanding at a steady CAGR of about 6.0% during the forecast period. This growth is primarily driven by rising demand from the pharmaceutical industry, where PVP is widely used as a tablet binder, solubilizer, and stabilizer, along with increasing consumption in cosmetics and personal care products…
More Releases for Regulatory
Medical Device Regulatory Affairs Market Medical Device Regulatory Affairs Marke …
"Medical Device Regulatory Affairs Market" in terms of revenue was estimated to be worth $ 6.7 billion in 2024 and is poised to reach $ 18.3 billion by 2034, growing at a CAGR of 10.8% from 2025 to 2034 according to a new report by InsightAce Analytic.
Request For Free Sample Pages:
https://www.insightaceanalytic.com/request-sample/1913
Latest Drivers Restraint and Opportunities Market Snapshot:
Key factors influencing the global medical device regulatory…
Medical Device & IVD Regulatory Affairs Outsourcing Market: Navigating Regulator …
Global healthcare landscape, the Medical Device & IVD Regulatory Affairs Outsourcing Market has emerged as a critical component ensuring the safe and compliant introduction of medical devices and in-vitro diagnostic products to the market. As the industry witnesses significant shifts and challenges, here's an in-depth analysis of the current trends, dynamics, and future prospects within this market segment.
Download sample PDF copy of report: https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=79264&utm_source=OpenPR_Ajay&utm_medium=OpenPR
Impact of COVID-19 on European Regulations
The outbreak of…
Regulatory Writing Market - Clear, Concise, Compliant: Redefining Regulatory Wri …
Newark, New Castle, USA - new report, titled Regulatory Writing Market The report has been put together using primary and secondary research methodologies, which offer an accurate and precise understanding of the Regulatory Writing market. Analysts have used a top-down and bottom-up approach to evaluate the segments and provide a fair assessment of their impact on the global Regulatory Writing market. The report offers an overview of the market, which…
Complex Regulatory Frameworks
It is challenging for new entrants to enter the FinTech industry because of its complex regulatory framework. All FinTech companies must comply with compliance requirements even before they begin operations, which increases their costs and creates a significant barrier for startups. While regulations are needed to protect consumers, a number of existing laws are slowing down the growth of many Indian FinTech companies, thereby extending their time to reach the…
South Africa Upstream Fiscal and Regulatory Report 2017 - Pending Legislation Cr …
Presented report, South Africa Upstream Fiscal and Regulatory Report 2017 - Pending Legislation Creates Regulatory Uncertainty, presents the essential information relating to the terms which govern investment into South Africa’s upstream oil and gas sector. The report sets out in detail the contractual framework under which firms must operate in the industry, clearly defining factors affecting profitability and quantifying the state’s take from hydrocarbon production. Considering political, economic and industry…
Regulatory Affairs Outsourcing Market (Services - Regulatory Submissions, Clinic …
This research study analyzes the market for regulatory affairs outsourcing services in terms of revenue (US$ Mn). The stakeholders of this report comprises the clinical research organizations. The global regulatory affairs outsourcing market has been broadly segmented on the basis of services (Regulatory Submissions, Clinical Trial Applications and Product Registrations, Regulatory Writing and Publishing, Regulatory Consulting and Legal Representation and others regulatory affairs, and Geography (North America, Europe, Asia Pacific,…