Press release
Medical Device Testing Market is Expected to Grow USD 22.0 Billion by 2033 | CAGR 5.47% During 2025-2033
IMARC Group, a leading market research company, has recently released a report titled "Medical Device Testing Market Report by Service (Testing Services, Inspection Services, and Others), Type (In-House, Outsourced), Testing Type (Biocompatibility Testing, Chemistry Testing, Bio-Burden Determination, Anti-Microbial Activity and Sterility Testing, and Others), Device Class (Class I, Class II, Class III), Device Type (Implantable Medical Devices, Non-Active Medical Devices, In-Vitro Diagnostic Medical Devices, Ophthalmic Medical Devices, and Others), and Region 2025-2033". The study provides a detailed analysis of the industry, including the global medical device testing market share, trends, size, and industry trends forecast. The report also includes competitor and regional analysis and highlights the latest advancements in the market.Report Highlights:
How Big Is the Medical Device Testing Market?
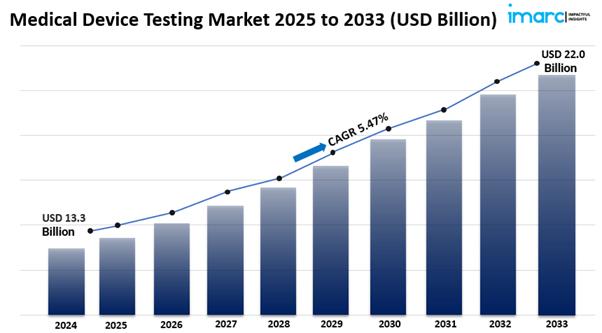
The global medical device testing market size reached USD 13.3 Billion in 2024. Looking forward, IMARC Group expects the market to reach USD 22.0 Billion by 2033, exhibiting a growth rate (CAGR) of 5.47% during 2025-2033. The imposition of strict regulatory requirements, significant technological advancements, growing emphasis on patient safety, increasing penetration of medical device manufacturer into emerging markets, rising complexity of medical devices, and ongoing competitive pressure are some of the major factors propelling the market.
Request to Get the Sample Report:
https://www.imarcgroup.com/medical-device-testing-market/requestsample
Market Dynamics of the Medical Device Testing Market:
• Increasing Regulatory Scrutiny and Compliance Requirements
Global regulatory bodies, such as the FDA (U.S.) and EMA (Europe), are continuously strengthening their oversight of medical devices. This heightened scrutiny stems from past product recalls and safety concerns, leading to more rigorous approval processes. Manufacturers are now mandated to conduct extensive testing, encompassing biocompatibility, performance, and clinical evaluation, to ensure device safety and efficacy. This escalating demand for comprehensive testing services is fostering a surge in collaborations between device manufacturers and specialized testing laboratories. These partnerships are crucial for advancing research into new testing methodologies and meeting evolving, complex regulatory standards.
• Technological Advancements in Testing Methods
Innovation in testing technologies is revolutionizing the medical device testing services market. The integration of Artificial Intelligence (AI), Machine Learning (ML), and automation is making medical device testing processes faster, more accurate, and cost-effective. AI, for instance, can analyze vast datasets to identify subtle patterns that traditional methods might overlook, significantly enhancing efficiency and reliability for medical device testing services. Furthermore, the development of advanced in vitro tests and simulations is reducing the reliance on animal testing, aligning with both ethical considerations and regulatory preferences in the medical device testing services market. These technological leaps not only expedite the testing phase but also accelerate time-to-market for devices, providing manufacturers with a competitive edge as the demand for novel medical devices continues to rise, further boosting the medical device testing services market.
• Growth of Personalized Medicine and Customized Devices
The burgeoning field of personalized medicine is profoundly impacting the medical device testing market, presenting both opportunities and challenges. As healthcare transitions towards treatments tailored to individual patients, the need for customized medical devices is rapidly expanding. These bespoke devices necessitate rigorous testing to ensure their safety and effectiveness for diverse patient profiles, considering factors like genetics, environment, and lifestyle. Consequently, testing laboratories are challenged to develop new methodologies capable of incorporating individual patient data into their evaluations. This imperative is fostering increased cooperation among manufacturers, healthcare providers, and testing labs, all striving to establish robust testing ecosystems that enhance patient outcomes and satisfaction in the era of personalized healthcare.
Get Discount On The Purchase Of This Report: https://www.imarcgroup.com/checkout?id=3343&method=1670
Medical Device Testing Market Report Segmentation:
Service Segments:
• Testing Services
• Inspection Services
• Others
The market is divided into testing services, inspection services, and others, with testing services being the largest segment.
Type Segments:
• In-House
• Outsourced
The market includes in-house and outsourced testing, where in-house is the dominant segment.
Testing Type Segments:
• Biocompatibility Testing
• Chemistry Testing
• Bio-Burden Determination
• Anti-Microbial Activity and Sterility Testing
• Others
This segment covers biocompatibility testing, chemistry testing, bio-burden determination, anti-microbial activity, and sterility testing, among others, with anti-microbial activity and sterility testing holding the largest market share.
Device Class Segments:
• Class I
• Class II
• Class III
The market is categorized into class I, class II, and class III devices, with class II representing the largest segment.
Device Type Segments:
• Implantable Medical Devices
• Non-Active Medical Devices
• In-Vitro Diagnostic Medical Devices
• Ophthalmic Medical Devices
• Others
This includes implantable medical devices, non-active medical devices, in-vitro diagnostic medical devices, ophthalmic medical devices, and others, reflecting a diverse range of device categories.
Regional Markets:
• North America
o United States
o Canada
• Asia-Pacific
o China
o Japan
o India
o South Korea
o Australia
o Indonesia
o Others
• Europe
o Germany
o France
o United Kingdom
o Italy
o Spain
o Russia
o Others
• Latin America
o Brazil
o Mexico
o Others
• Middle East and Africa
The report analyzes major regions including North America, Asia-Pacific, Europe, Latin America, and the Middle East and Africa, highlighting significant market dynamics in each area.
Competitive Landscape with Key Players:
The competitive landscape of the medical device testing market size has been studied in the report with the detailed profiles of the key players operating in the market.
Some of These Key Players Include:
• Bureau Veritas
• Charles River Laboratories
• Element Materials Technology
• EndoLab Mechanical Engineering GmbH
• Eurofins Scientific
• Gateway Analytical
• Intertek Group Plc
• Labcorp
• Medical Engineering Technologies Ltd.
• Medistri SA
• NAMSA
• Nelson Laboratories, LLC
• SGS SA
• TUV SUD
Ask Analyst for Customized Report:
https://www.imarcgroup.com/request?type=report&id=3343&flag=C
Key Highlights of the Report:
• Market Performance (2019-2024)
• Market Outlook (2025-2033)
• Market Trends
• Market Drivers and Success Factors
• Impact of COVID-19
• Value Chain Analysis
If you need specific information that is not currently within the scope of the report, we will provide it to you as a part of the customization.
About Us
IMARC Group is a leading market research company that offers management strategy and market research worldwide. We partner with clients in all sectors and regions to identify their highest-value opportunities, address their most critical challenges, and transform their businesses.
IMARC's information products include major market, scientific, economic and technological developments for business leaders in pharmaceutical, industrial, and high technology organizations. Market forecasts and industry analysis for biotechnology, advanced materials, pharmaceuticals, food and beverage, travel and tourism, nanotechnology and novel processing methods are at the top of the company's expertise.
Contact Us:
IMARC Group
134 N 4th St
Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Americas: +1-631-791-1145 | Europe & Africa: +44-753-713-2163 | Asia: +91-120-433-0800
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Medical Device Testing Market is Expected to Grow USD 22.0 Billion by 2033 | CAGR 5.47% During 2025-2033 here
News-ID: 4090825 • Views: …
More Releases from IMARC Group

Charcoal Production Plant DPR & Unit Setup 2026: Demand Analysis and Project Cos …
Setting up a charcoal production plant involves strategic planning, substantial capital investment, and comprehensive understanding of production technologies. This essential biomass fuel serves cooking, heating, metallurgical, and industrial applications. Success requires careful site selection, efficient carbonization processes, proper kiln systems, reliable wood and biomass sourcing, and compliance with environmental and forestry regulations to ensure profitable and sustainable operations.
IMARC Group's report, "Charcoal Production Plant Project Report 2026: Industry Trends, Plant Setup,…

Grease Manufacturing Plant DPR 2026: CapEx/OpEx Analysis with Profitability Fore …
Setting up a grease manufacturing plant involves strategic planning, substantial capital investment, and comprehensive understanding of production technologies. This essential lubricant serves automotive, industrial machinery, and heavy equipment applications. Success requires careful site selection, efficient blending and mixing processes, quality control systems, reliable raw material sourcing from base oil and thickener suppliers, and compliance with environmental and safety regulations to ensure profitable and sustainable operations.
IMARC Group's report, "Grease Production Plant…

Tequila Manufacturing Plant Cost 2026: CapEx, OpEx & ROI Analysis
Setting up a Tequila Manufacturing Plant positions investors in one of the most stable and essential segments of the premium spirits and alcoholic beverages value chain, backed by sustained global growth driven by rising international demand for authentic agave-based spirits, increasing consumer preference for premium and super-premium heritage-driven beverages, expanding consumption across hospitality, retail, and export channels, and the dual-benefit advantages of strong brand loyalty combined with high-margin product differentiation.…

Prefabricated Building and Structural Steel Manufacturing Plant Cost Analysis Re …
Setting up a prefabricated building and structural steel manufacturing plant positions investors within one of the most dynamic and infrastructure-driven segments of the global construction and industrial manufacturing sector, supported by accelerating urbanization, expanding industrial corridors, and rising demand for fast-track, cost-efficient building solutions across residential, commercial, and industrial projects.
Prefabricated structures and structural steel components play a critical role in modern construction by enabling reduced project timelines, improved quality…
More Releases for Device
Medical Device Regulatory Affairs Market Medical Device Regulatory Affairs Marke …
"Medical Device Regulatory Affairs Market" in terms of revenue was estimated to be worth $ 6.7 billion in 2024 and is poised to reach $ 18.3 billion by 2034, growing at a CAGR of 10.8% from 2025 to 2034 according to a new report by InsightAce Analytic.
Request For Free Sample Pages:
https://www.insightaceanalytic.com/request-sample/1913
Latest Drivers Restraint and Opportunities Market Snapshot:
Key factors influencing the global medical device regulatory…
Surge In Wireless Device Usage Boosts Wireless Audio Device Market Driving Marke …
Stay ahead with our updated market reports featuring the latest on tariffs, trade flows, and supply chain transformations.
How Large Will the Wireless Audio Device Market Size By 2025?
In recent years, there has been remarkable growth in the wireless audio device market size. The market, which is projected to expand from $41.85 billion in 2024 to $52.37 billion in 2025, boasts a compound annual growth rate (CAGR) of 25.1%. Factors contributing…
Anti-snoring Device Market - Quiet Nights, Restful Sleep: Anti-snoring Device In …
Newark, New Castle, USA: The "Anti-snoring Device Market" provides a value chain analysis of revenue for the anticipated period from 2023 to 2031. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors.
Anti-snoring Device Market: https://www.growthplusreports.com/report/antisnoring-device-market/8931
This latest report researches the industry structure, sales, revenue,…
Global Watch Clock Measuring Device Market | Watch Clock Measuring Device Indust …
Watch, clock and measuring device market comprises of the sales of watch, clock, measuring device & related services to measure the time and physical quantity. Watch is portable timepiece, which is worn by people around the wrist, attached by a strap. Clock is a device used to measure and indicate time, using the pointers moving over a dial. Measuring device is an instrument used for measuring the various parameters in…
Peripheral Vascular Device Market Size, Peripheral Vascular Device Market Share, …
Global Peripheral Vascular Device Market Size is observed to gain traction owing to the factors such as increasing research and development for developing several new product, and rising funding by the private organizations.
Request for Sample of This Research Report @ https://bit.ly/2xjOKpC
Top Key Player:-
Abbott Laboratories
Braun Melsungen AG
Boston Scientific Corporation
R. Brad, Inc.
Cardinal Health, Inc.
Medtronic plc.
Cook Medical, Inc.
Teruma Corporation
Jude Medical, Inc.
The Spectranetics Corporation
Volcano Corporation
Peripheral vascular disorder (PVD) is a blood circulation disorder…
Medical Device Technologies Market - The Evolution of Medical Device Technologie …
The global medical device technologies market is anticipated to be boosted by various well-known players in the market. Some of these players that are dealing with the manufacturing of in vitro diagnostic devices hold a significant share in the global market. Whereas, the small market players are emerging from several developing nations, looking to set their foot in the market. Such measures are foreseen to change the market scenario in…