Press release
Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Clinical Trials Analysis 2025: EMA, PDMA, FDA Approvals, Therapies, Medication, Mechanism of Action, Route of Administration by DelveInsight
Chronic Inflammatory Demyelinating Polyneuropathy Companies such as Takeda, Sanofi, Johnson & Johnson Services, Immunovant, UCB S.A., GeNeuro, Nanjing IASO Biotherapeutics, Bioasis Technologies Inc., and others.(Las Vegas, Nevada, United States) As per DelveInsight's assessment, globally, Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) pipeline constitutes 5+ key companies continuously working towards developing 6+ Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) treatment therapies, analysis of Clinical Trials, Therapies, Mechanism of Action, Route of Administration, and Developments analyzes DelveInsight.
"Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Pipeline Insight, 2025"report by DelveInsight outlines comprehensive insights into the present clinical development scenario and growth prospects across the Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Market.
The Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Pipeline report embraces in-depth commercial and clinical assessment of the pipeline products from the pre-clinical developmental phase to the marketed phase. The report also covers a detailed description of the drug, including the mechanism of action of the drug, clinical studies, NDA approvals (if any), and product development activities comprising the technology, collaborations, mergers acquisition, funding, designations, and other product-related details.
Request for Sample Report here @ Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Pipeline Outlook [https://www.delveinsight.com/report-store/chronic-inflammatory-demyelinating-polyneuropathy-cidp-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=apr]
Some of the key takeaways from the Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Pipeline Report:
* CIDP Companies across the globe are diligently working toward developing novel Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) treatment therapies with a considerable amount of success over the years.
* In April 2025, argenx SE (Euronext & Nasdaq: ARGX), a global immunology company committed to improving the lives of people suffering from severe autoimmune diseases, today announced that the U.S. Food and Drug Administration (FDA) approved a new option for patients to self-inject VYVGART Registered Hytrulo with a prefilled syringe (efgartigimod alfa and hyaluronidase-qvfc) for the treatment of adult patients with generalized myasthenia gravis (gMG) who are anti-acetylcholine receptor (AChR) antibody positive and adult patients with chronic inflammatory demyelinating polyneuropathy (CIDP).
* In November 2024:- Octapharma- Multicenter, Prospective, Double-Blinded, Parallel Group, Randomized Phase III Study to Evaluate Safety and Efficacy of Different PANZYGA Dose Regimens in Pediatric Chronic Inflammatory Demyelinating Polyradiculoneuropathy (CIDP) Patients
* June 2024:- Immunovant Sciences GmbH- A Phase 2b, Multi-center, Randomized, Quadruple-blind, Placebo-controlled Study of Batoclimab Treatment in Adult Participants With Active Chronic Inflammatory Demyelinating Polyneuropathy (CIDP).
* June 2024:- Janssen Research & Development LLC- Phase 2/3, Multistage, Multicenter, Randomized, Double-Blind, Placebo-Controlled Parallel Group Withdrawal Study to Evaluate the Efficacy and Safety of Nipocalimab Administered to Adults With Chronic Inflammatory Demyelinating Polyneuropathy (CIDP).
* The leading Chronic Inflammatory Demyelinating Polyneuropathy Companies such as Takeda, Sanofi, Johnson & Johnson Services, Immunovant, UCB S.A., GeNeuro, Nanjing IASO Biotherapeutics, Bioasis Technologies Inc., and others.
* Promising Chronic Inflammatory Demyelinating Polyneuropathy Therapies such as Riliprubart, Batoclimab 680 mg, SAR445088, Panzyga, Efgartigimod PH20 SC, Nipocalimab, IgPro10, Immunoadsorption, TAK-771, and others.
Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Overview
Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) is a rare, immune-mediated neurological disorder characterized by progressive weakness and impaired sensory function in the legs and arms. It arises when the body's immune system mistakenly attacks the myelin sheath - the protective covering of the peripheral nerves - leading to demyelination and nerve damage. Unlike Guillain-Barre Syndrome (GBS), which is acute, CIDP follows a chronic course, typically evolving over at least eight weeks.
The exact cause of CIDP is unknown, but it is believed to involve an autoimmune response possibly triggered by infections or other environmental factors in genetically susceptible individuals. Patients often present with symmetrical limb weakness, numbness, tingling, and diminished reflexes. If untreated, CIDP can result in significant disability due to persistent nerve damage.
Diagnosis involves clinical evaluation, nerve conduction studies showing demyelination, cerebrospinal fluid analysis often revealing elevated protein levels, and sometimes nerve biopsy. Treatment focuses on controlling immune activity and includes corticosteroids, intravenous immunoglobulin (IVIg), and plasma exchange, which can significantly improve or stabilize symptoms. With timely therapy, many patients achieve partial or complete recovery, though relapses are common. Long-term management is essential to monitor disease progression and adjust treatment strategies.
Get a Free Sample PDF Report to know more about Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Pipeline Therapeutic Assessment- Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Treatment Drugs [https://www.delveinsight.com/report-store/chronic-inflammatory-demyelinating-polyneuropathy-cidp-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=apr]
Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Route of Administration
Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) pipeline report provides the therapeutic assessment of the pipeline drugs by the Route of Administration. Products have been categorized under various ROAs, such as
* Oral
* Intravenous
* Subcutaneous
* Parenteral
* Topical
Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Molecule Type
Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Products have been categorized under various Molecule types, such as
* Recombinant fusion proteins
* Small molecule
* Monoclonal antibody
* Peptide
* Polymer
* Gene therapy
Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Pipeline Therapeutics Assessment
* Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Assessment by Product Type
* Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) By Stage and Product Type
* Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Assessment by Route of Administration
* Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) By Stage and Route of Administration
* Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Assessment by Molecule Type
* Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) by Stage and Molecule Type
DelveInsight's Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Report covers around 6+ products under different phases of clinical development like-
* Late-stage products (Phase III)
* Mid-stage products (Phase II)
* Early-stage product (Phase I)
* Pre-clinical and Discovery stage candidates
* Discontinued & Inactive candidates
* Route of Administration
Further Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) product details are provided in the report. Download the Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) pipeline report to learn more about the emerging Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) therapies [https://www.delveinsight.com/sample-request/chronic-inflammatory-demyelinating-polyneuropathy-cidp-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=apr]
Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Pipeline Analysis:
The Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) pipeline report provides insights into
* The report provides detailed insights about companies that are developing therapies for the treatment of Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) with aggregate therapies developed by each company for the same.
* It accesses the Different therapeutic candidates segmented into early-stage, mid-stage, and late-stage of development for Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Treatment.
* Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) key companies are involved in targeted therapeutics development with respective active and inactive (dormant or discontinued) projects.
* Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Drugs under development based on the stage of development, route of administration, target receptor, monotherapy or combination therapy, a different mechanism of action, and molecular type.
* Detailed analysis of collaborations (company-company collaborations and company-academia collaborations), licensing agreement and financing details for future advancement of the Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) market.
Download Sample PDF Report to know more about Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) drugs and therapies- Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Medication and Companies [https://www.delveinsight.com/sample-request/chronic-inflammatory-demyelinating-polyneuropathy-cidp-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=apr]
Scope of Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Pipeline Drug Insight
* Coverage: Global
* The leading Chronic Inflammatory Demyelinating Polyneuropathy Companies such as Takeda, Sanofi, Johnson & Johnson Services, Immunovant, UCB S.A., GeNeuro, Nanjing IASO Biotherapeutics, Bioasis Technologies Inc., and others.
* Promising Chronic Inflammatory Demyelinating Polyneuropathy Therapies such as Riliprubart, Batoclimab 680 mg, SAR445088, Panzyga, Efgartigimod PH20 SC, Nipocalimab, IgPro10, Immunoadsorption, TAK-771, and others.
* Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Therapeutic Assessment: Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) current marketed and Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) emerging therapies
* Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Market Dynamics: Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) market drivers and Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) market barriers
Request for Sample PDF Report for Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Pipeline Assessment and clinical trials @ Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) FDA Approvals and Clinical Advancements [https://www.delveinsight.com/sample-request/chronic-inflammatory-demyelinating-polyneuropathy-cidp-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=apr]
Table of Contents
1. Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Report Introduction
2. Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Executive Summary
3. Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Overview
4. Chronic Inflammatory Demyelinating Polyneuropathy (CIDP)- Analytical Perspective In-depth Commercial Assessment
5. Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Pipeline Therapeutics
6. Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Late Stage Products (Phase II/III)
7. Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Mid Stage Products (Phase II)
8. Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Early Stage Products (Phase I)
9. Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Preclinical Stage Products
10. Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Therapeutics Assessment
11. Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Inactive Products
12. Company-University Collaborations (Licensing/Partnering) Analysis
13. Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Companies
14. Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Key Products
15. Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Unmet Needs
16 . Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Market Drivers and Barriers
17. Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Future Perspectives and Conclusion
18. Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Analyst Views
19. Appendix
20. About DelveInsight
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports Pharma companies by providing comprehensive end-to-end solutions to improve their performance. It also offers Healthcare Consulting Services, which benefits in market analysis to accelerate business growth and overcome challenges with a practical approach.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Ankit Nigam
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=chronic-inflammatory-demyelinating-polyneuropathy-cidp-clinical-trials-analysis-2025-ema-pdma-fda-approvals-therapies-medication-mechanism-of-action-route-of-administration-by-delveinsight]
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Albany
State: New York
Country: United States
Website: https://www.delveinsight.com/consulting/partner-identification-services
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Clinical Trials Analysis 2025: EMA, PDMA, FDA Approvals, Therapies, Medication, Mechanism of Action, Route of Administration by DelveInsight here
News-ID: 4087392 • Views: …
More Releases from ABNewswire
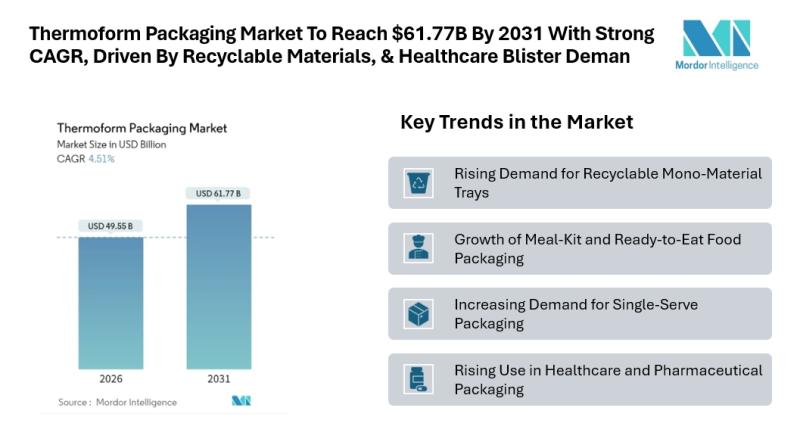
Thermoform Packaging Market to Reach $61.77B By 2031 With A Strong CAGR, Driven …
Mordor Intelligence has published a new report on the thermoform packaging market, offering a comprehensive analysis of trends, growth drivers, and future projections.
Outlook of the Thermoform Packaging Market
According to Mordor Intelligence, the thermoform packaging market size is estimated at USD 49.55 billion in 2026, growing from USD 47.41 billion in 2025 and projected to reach USD 61.77 billion by 2031, registering a CAGR of 4.51% during the forecast period. This…

Florida Physician Launches 2026 "Hardware and Software" Men's Longevity Initiati …
LifeWellMD in Palm Beach and Port St. Lucie, Florida, has launched a 2026 Men's Longevity Initiative that treats the male body like a highperformance system-fixing "hardware" with regenerative and vascular therapies, upgrading "software" with hormone and sleep optimization, and in select cases installing a "new operating system" using advanced cellular options. The program gives men a clear roadmap to improve energy, recovery, focus, and longterm health-span.
Port St. Lucie, FL -…

Tessa Belanger's "PASS THE SAGE" presents 'Stories from the fire, teachings from …
PASS THE SAGE: Stories from the Fire, Teachings from the Smoke is a stirring new anthology from Algonquin author Tessa Belanger that gathers powerful Indigenous voices into one luminous collection. Rooted in ceremony, shaped by community, and told with unflinching honesty. PASS THE SAGE invites readers to sit by the fire, burn our medicines and listen deeply while you carry the teachings forward.
Born from workshops, circles, and lived experience, this…

Prestige Blinds of Coral Springs Named Top Rated Window Treatment Company on Goo …
Prestige Blinds of Coral Springs, a leading provider of custom blinds, shades, and window treatments in Coral Springs, Florida, has earned recognition as a Top Rated Company on Google, based on outstanding customer reviews and five-star ratings.
This achievement reflects the company's reputation as a trusted local window treatment specialist serving Coral Springs and surrounding Broward County areas, including Parkland, Coconut Creek, Margate, Tamarac, and Deerfield Beach. Customers consistently highlight Prestige…
More Releases for Chronic
Chronic Pain Treatment Market - Relieving Chronic Pain: Discover the Latest Inno …
Newark, New Castle, USA: The "Chronic Pain Treatment Market" provides a value chain analysis of revenue for the anticipated period from 2022 to 2030. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors
Chronic Pain Treatment Market: https://www.growthplusreports.com/report/chronic-pain-treatment-market/8030
This latest report researches the industry structure,…
Refractory Chronic Cough Therapeutics Market - Silencing the Cough: Innovative T …
Newark, New Castle, USA: The "Refractory Chronic Cough Therapeutics Market" provides a value chain analysis of revenue for the anticipated period from 2022 to 2030. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors
Refractory Chronic Cough Therapeutics Market: https://www.growthplusreports.com/report/refractory-chronic-cough-therapeutics-market/7991
This latest report researches the…
Chronic Pulmonary Aspergillosis Drugs Market - Revitalizing Lungs: Revolutionary …
Newark, New Castle, USA - new report, titled Chronic Pulmonary Aspergillosis Drugs Market The report has been put together using primary and secondary research methodologies, which offer an accurate and precise understanding of the Chronic Pulmonary Aspergillosis Drugs market. Analysts have used a top-down and bottom-up approach to evaluate the segments and provide a fair assessment of their impact on the global Chronic Pulmonary Aspergillosis Drugs market. The report offers…
Chronic Hepatitis Therapeutics Market - Empowering Liver Health, Defying Chronic …
Newark, New Castle, USA - new report, titled Chronic Hepatitis Therapeutics Market The report has been put together using primary and secondary research methodologies, which offer an accurate and precise understanding of the Chronic Hepatitis Therapeutics market. Analysts have used a top-down and bottom-up approach to evaluate the segments and provide a fair assessment of their impact on the global Chronic Hepatitis Therapeutics market. The report offers an overview of…
Chronic Phase Chronic Myeloid Leukemia Market to Witness Growth by 2032, Estimat …
DelveInsight's "Chronic Phase Chronic Myeloid Leukemia Market Insights, Epidemiology, and Market Forecast-2032" report delivers an in-depth understanding of Chronic Phase Chronic Myeloid Leukemia , historical and forecasted epidemiology as well as the Chronic Phase Chronic Myeloid Leukemia market trends in the United States, EU5 (Germany, Spain, Italy, France, and United Kingdom), and Japan.
The Chronic Phase Chronic Myeloid Leukemia market report provides current treatment practices, emerging drugs, the market share…
Boot chronic pain
For immediate release
Due to our rushed lives and stressful environment, many people are suffering from chronic pain and fatigue.
We feel permanently tired and are barely able to get through our normal day. One is able to change this status when following the guidelines the book "Tired of being Tired to the point of being Gatvol (Fed Up). Some people will need a team of medical practitioners to help them…
