Press release
Preeclampsia Market Epidemiology | In-Market Drugs Sales, Pipeline Therapies and Regional Outlook 2025-2035

Preeclampsia Market Epidemiology | In-Market Drugs Sales, Pipeline Therapies and Regional Outlook 2025-2035
The preeclampsia market in 2025 is showing a dynamic growth trend based on the increasing prevalence of preeclampsia, awareness generation, and swift technological advancements in treatment, monitoring, and diagnosis. Preeclampsia is a pregnancy-related disorder characterized by hypertension and later damage to organs, which is a huge risk for the mother and the fetus. With maternal health on everyone's agenda, the preeclampsia market is moving up to meet this need through innovation, improved pathways of care, and enhanced therapy options.
Request for a sample copy of the report: https://www.imarcgroup.com/preeclampsia-market/requestsample
Market Trends
Preeclampsia in 2025 will be emphasizing some important trends for maternal healthcare. Early detection continues to be central and is further enhanced by improved incorporation of routine screening at antenatal consultations. Biomarker testing advancements, such as proteins like placental growth factor (PlGF) and soluble fms-like tyrosine kinase-1 (sFlt-1), enable much more accurate and rapid preeclampsia risk assessment. These assays are currently being extensively thought of as a routine part of prenatal care so that clinicians can identify high-risk pregnancies more readily and early on than has been conventionally practiced with blood pressure checks and determination of proteinuria.
Home health monitoring is increasingly gaining traction as secondary care models expand. Pregnant women at risk can now use connected blood pressure monitors and wearables to transmit real-time readings to their physicians. This remote care approach facilitates rapid intervention and eliminates unnecessary clinic visits, raising convenience and safety for pregnant women.
Pharmacological advancement is also important. For example, aspirin prophylaxis, already recognized as effective and cheap for preventing preeclampsia among groups at risk, is now being supplemented with new methods for treatment. Other treatment advances include low-molecular-weight heparin, specific antihypertensive agents, and even biologics designed to address placental dysfunction. The fact that these drugs are now in clinical trials shows how treatment is gradually becoming individualized.
Reasons for Rising Incidence
The globally rising incidence of preeclampsia can be described by several interrelated factors. With maternal age at first pregnancy on the rise worldwide, more and more women are getting pregnant at an older age. This is linked with an increased incidence of hypertension, obesity, and metabolic disorders-all of which are established risk factors for preeclampsia. In addition, enhanced diagnosis aided by complete prenatal care and improved availability of medical assistance in neglected areas have all contributed towards an increased demand for accepted prevalence.
There is an increasing contribution of lifestyle factors also. Physical inactivity, poor diet, and rising obesity rates cause elevated blood pressure and metabolic stress during pregnancy. The convergence of these demographic and lifestyle trends has placed preeclampsia prevention and management at the forefront of healthcare systems and policymaking priorities.
Buy the full Preeclampsia Epidemiology Report: https://www.imarcgroup.com/checkout?id=7508&method=809
The report also provides a detailed analysis of the current preeclampsia marketed drugs and late-stage pipeline drugs.
In-Market Drugs
Drug Overview
Mechanism of Action
Regulatory Status
Clinical Trial Results
Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
Drug Overview
Mechanism of Action
Regulatory Status
Clinical Trial Results
Drug Uptake and Market Performance
Competitive Landscape:
The competitive landscape of the preeclampsia market has been studied in the report with the detailed profiles of the key players operating in the market.
Analysis Covered Across Each Country
Historical, current, and future epidemiology scenario
Historical, current, and future performance of the preeclampsia market
Historical, current, and future performance of various therapeutic categories in the market
Sales of various drugs across the preeclampsia market
Reimbursement scenario in the market
In-market and pipeline drugs
7 Major Countries Covered
United States
Germany
France
United Kingdom
Italy
Spain
Japan
Contact US:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
United States: +1-631-791-1145
About Us:
IMARC Group is a global management consulting firm that helps the world's most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Preeclampsia Market Epidemiology | In-Market Drugs Sales, Pipeline Therapies and Regional Outlook 2025-2035 here
News-ID: 4084714 • Views: …
More Releases from IMARC Group
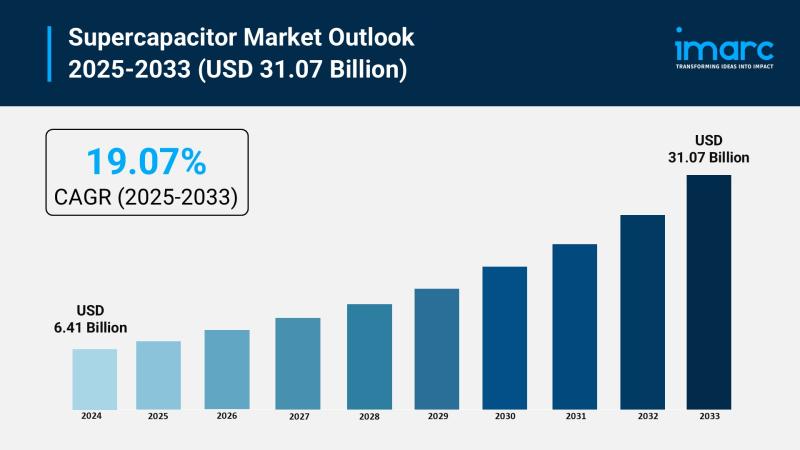
Supercapacitor Market Size to Reach $31.07B by 2033: Trends & Opportunities
Market Overview:
The supercapacitor market is experiencing rapid growth, driven by electrification of automotive systems, renewable energy and grid stabilization, and expansion of industrial automation and robotics. According to IMARC Group's latest research publication, "Supercapacitor Market Size, Share, Trends and Forecast by Product Type, Module Type, Material Type, End Use Industry, and Region, 2025-2033", the global supercapacitor market size was valued at USD 6.41 Billion in 2024. Looking forward, IMARC Group…
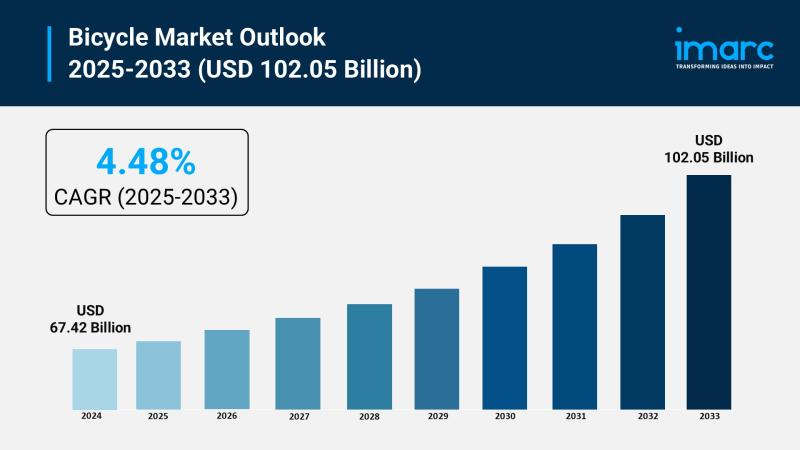
Bicycle Market Size to Surpass $102.05B by 2033: Growth & Insights
Market Overview:
The bicycle market is experiencing rapid growth, driven by global expansion of cycling infrastructure, rising health consciousness and preventative wellness, and technological advancements in e-bike propulsion. According to IMARC Group's latest research publication, "Bicycle Market Size, Share, Trends and Forecast by Type, Technology, Price, Distribution Channel, End User, and Region, 2025-2033", The global bicycle market size was valued at USD 67.42 Billion in 2024. Looking forward, IMARC Group estimates…
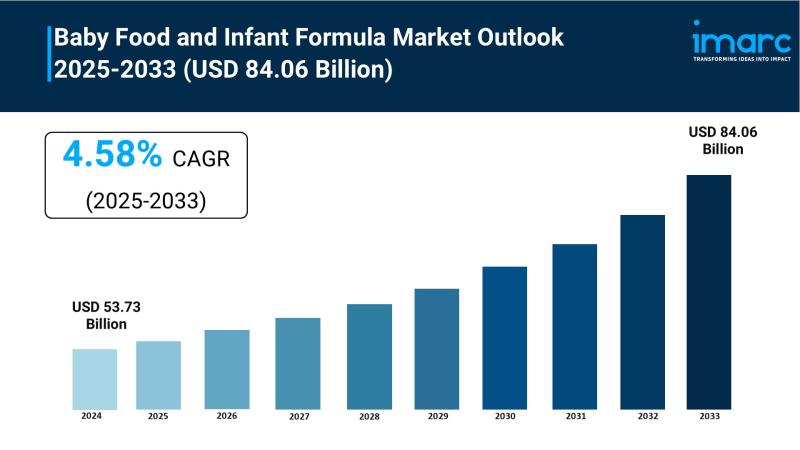
Baby Food and Infant Formula Market to Reach USD 84.06 Billion by 2033, Growing …
Market Overview:
The Baby Food and Infant Formula Market is experiencing steady expansion, driven by Increasing Awareness of Nutritional Needs for Infants, Rising Number of Working Women, and Technological Advancements and Product Innovation. According to IMARC Group's latest research publication, "Baby Food and Infant Formula Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2025-2033", The global baby food and infant formula market size reached USD 53.73 Billion in 2024.…
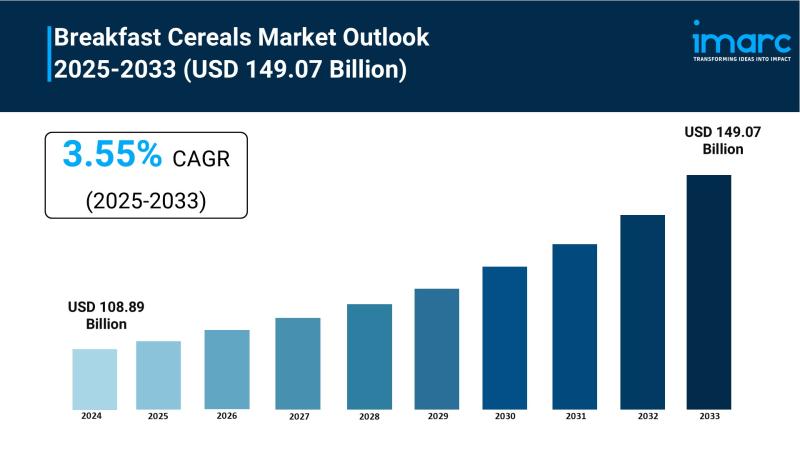
Breakfast Cereals Market to Reach USD 149.07 Billion by 2033, Growing at a CAGR …
Market Overview:
The Breakfast Cereals Market is experiencing rapid growth, driven by Health and Wellness Awareness, Busy Lifestyles and On-the-Go Demand and Rising Disposable Incomes and Global Market Expansion . According to IMARC Group's latest research publication, "Breakfast Cereals Market : Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2025-2033", The global breakfast cereals market size was valued at USD 108.89 Billion in 2024. Looking forward, IMARC Group estimates…
More Releases for Drug
Injectable Drug Delivery Market Injectable Drug Delivery Market
Leading market research firm SkyQuest Technology Group recently released a study titled ' Injectable Drug Delivery Market Global Size, Share, Growth, Industry Trends, Opportunity and Forecast 2024-2031,' This study Injectable Drug Delivery report offers a thorough analysis of the market, as well as competitor and geographical analysis and a focus on the most recent technological developments. The research study on the Injectable Drug Delivery Market extensively demonstrates existing and upcoming…
Global Advanced Drug Delivery Systems Market Size - By Product Type(Oral Drug De …
Market Overview and Report Coverage
Advanced Drug Delivery Systems (ADDS) refer to innovative technologies designed to improve the administration and efficacy of therapeutics, enhancing the way medications are delivered to targeted areas within the body. These systems aim to optimize treatment outcomes by increasing the bioavailability, reducing side effects, and facilitating controlled drug release. Employing methods such as nanoparticles, liposomes, and implantable pumps, ADDS are revolutionizing personalized medicine and expanding therapeutic…
Global Cancer Antibody Drug Conjugate Market Size, Drug Sales, Drug Dosage, Pric …
Global Cancer Antibody Drug Conjugate Market Size, Drug Sales, Drug Dosage, Price, and Clinical Trials Outlook 2029 Report Highlights:
* Global Antibody Drug Conjugates Market Opportunity: > 40 Billion By 2029
* Global and Regional Antibody Drug Conjugate Market Insight
* Approved Drugs Sales Insight Global and Regional, Yearly and Quarterly, 2019 -2023
* Approved Antibody Drug Conjugates - Availability, Dosage and Price Insight
* Insight On Antibody Drug Conjugates In Clinical Trials: > 550…
Alcohol Testing And Drug Testing Equipment Market 2025 Segmentation, Application …
Market Study Report, LLC, has compiled an exhaustive research study of the ‘Alcohol Testing And Drug Testing Equipment market’, detailing every single market driver and intricately analyzing the business vertical. This ‘Alcohol Testing And Drug Testing Equipment market’ study will aid in seeking out new business opportunities and fine-tuning existing marketing strategies through insights regarding SWOT analysis, market valuation, competitive spectrum, regional share, and revenue predictions.
Alcohol abuse and drug…
How much Diabetes Drug Market Impact Worldwide Medical Drug Industry?
Diabetes Drug Market From an insight perspective, the market report focuses on various levels of analyses — industry analysis, market rank analysis, and company profiles, which together comprise and discuss basic views on the competitive landscape, high-growth regions, and countries as well as their respective regulatory policies, Types ,Applications and opportunities in the market.
Diabetes is a metabolic disorder in which the body glucose level is elevated. There are two types of diabetes…
Hepatitis Drug Market Hepatitis Drug Clinical Pipeline Report 2023
For Report Sample Contact: neeraj@kuickresearch.com or +91-11-47067990
Report Table of Contents
1. Introduction to Hepatitis Disease
1.1 Prologue
1.1.1 History of Hepatitis
1.1.2 Causes of Hepatitis Disease
1.2 Types of Viruses which are Responsible for Hepatitis Disease
2. Global Prevalence of Hepatitis Infection
3. Available Drug Classes for Hepatitis Disease Treatment
3.1 Interferon Alfa Therapy
3.2 Protease Inhibitors Therapy
3.3 Polymerase…