Press release
GMP Storage Market Driven by Rising Demand for Regulatory Compliance and Product Safety
✅ GMP Storage Market: Ensuring Compliance and Safety in Regulated IndustriesThe GMP (Good Manufacturing Practices) storage market has emerged as a crucial component in sectors such as pharmaceuticals, biotechnology, healthcare, and food processing. These industries demand high standards of storage that comply with strict regulatory guidelines for temperature, humidity, contamination control, and traceability. As global regulations become more stringent and quality assurance takes center stage, the demand for compliant storage solutions continues to surge.
The market was valued at USD 3.5 billion in 2024 and is projected to reach USD 6.8 billion by 2032, growing at a CAGR of 8.7% during the forecast period. One of the key growth drivers is the rising pharmaceutical and biotech production capacity worldwide. Cold storage solutions, particularly for biologics and vaccines, dominate the market due to their critical need for temperature-sensitive environments. Regionally, North America leads the market, driven by advanced pharmaceutical infrastructure and strict FDA regulations promoting GMP compliance.
Get a Sample PDF Brochure of the Report (Use Corporate Email ID for a Quick Response): https://www.persistencemarketresearch.com/samples/33113
➤ Key Highlights from the Report
➤ The market is forecasted to grow at a CAGR of 8.7% from 2024 to 2032.
➤ North America holds the largest share due to stringent regulatory compliance and advanced R&D infrastructure.
➤ Cold storage leads as the dominant segment, driven by increasing demand for vaccine and biologic preservation.
➤ The pharmaceutical industry is the largest end-user segment in the GMP storage market.
➤ Asia-Pacific is expected to witness the fastest growth due to increased investments in pharmaceutical manufacturing.
➤ Key players are focusing on modular and customizable storage solutions to meet evolving GMP standards.
✅ What Is GMP Storage and Why Is It Important?
What is GMP storage and why is it essential in the pharmaceutical and biotech industries?
GMP storage refers to storage environments that comply with Good Manufacturing Practices-international standards that ensure products are consistently produced and controlled according to quality standards. This is especially critical in the pharmaceutical, biotechnology, and medical device sectors where improper storage can compromise the efficacy, safety, and integrity of the products.
GMP-compliant storage facilities are designed to control temperature, humidity, contamination, and light exposure. They also offer traceability, auditing capabilities, and automated inventory management, all crucial for regulatory compliance. For instance, storing vaccines at sub-zero temperatures or maintaining the stability of sensitive drugs requires precision that only GMP storage solutions can provide. In addition, these facilities often feature real-time monitoring, alert systems, and backup mechanisms to avoid product loss or degradation. As regulatory scrutiny increases globally, investing in GMP storage is not just a compliance measure-it is a business imperative.
✅ Market Segmentation: Diversification of Offerings to Cater to Varied Demands
The GMP storage market is segmented by product type, which includes cold storage units, ambient storage units, and controlled room temperature (CRT) storage. Among these, cold storage dominates due to the increasing use of temperature-sensitive products like biologics, vaccines, and clinical trial materials. The growth in biologic drug development has necessitated advanced storage environments that strictly adhere to temperature and humidity controls.
Based on end-user, the market is classified into pharmaceutical companies, biotech firms, contract development and manufacturing organizations (CDMOs), hospitals, and research laboratories. Pharmaceutical companies lead the segment due to the extensive regulatory requirements and the sheer volume of sensitive drugs that need GMP-compliant storage. CDMOs are also rapidly adopting GMP storage to align with outsourcing trends and meet client quality expectations.
✅ Regional Insights: Market Landscape Across Geographies
North America remains the largest regional market due to its robust pharmaceutical industry, strong R&D ecosystem, and stringent regulatory oversight from bodies such as the FDA. Major investments in clinical research and biologics manufacturing further support the demand for GMP storage infrastructure.
In contrast, Asia-Pacific is poised for the highest CAGR during the forecast period. Countries like China and India are heavily investing in pharmaceutical manufacturing and R&D to establish themselves as global hubs. This rapid industrialization and the rise in contract manufacturing have led to a sharp uptick in GMP storage demand in the region.
✅ Market Drivers, Restraints, and Opportunities
Market Drivers:
The primary driver for the GMP storage market is the growing need for regulatory compliance across pharmaceutical and biotech sectors. The increasing complexity of drugs, especially biologics, requires precise storage environments. Additionally, global expansion of pharmaceutical supply chains necessitates secure and standardized storage to maintain product integrity across borders.
Market Restraints:
Despite the promising growth, the market faces challenges such as high capital investment costs associated with building and maintaining GMP-compliant facilities. Regulatory compliance can be both time-consuming and expensive, particularly for small and mid-sized players. Additionally, a lack of skilled workforce for managing advanced storage systems can hinder operational efficiency.
Market Opportunities:
The surge in clinical trials and personalized medicine is creating opportunities for GMP storage solutions tailored for smaller batch sizes and shorter turnaround times. Another promising trend is the integration of digital monitoring technologies, enabling real-time tracking of temperature, humidity, and security. This is especially valuable for global supply chains needing visibility at all stages.
✅ Reasons to Buy the Report
☑️ In-depth analysis of global and regional GMP storage trends and growth projections
☑️ Comprehensive segmentation by product type, application, and end-user
☑️ Insights into emerging technologies and innovations driving market evolution
☑️ Competitive landscape with key player strategies and recent developments
☑️ Actionable recommendations to help stakeholders capitalize on high-growth opportunities
✅ Company Insights
✦ Thermo Fisher Scientific
✦ Avantor Inc.
✦ Azenta Life Sciences
✦ QStorage Solutions
✦ PHC Corporation
✦ Biostore UK Ltd
✦ Hamilton Company
✦ Haier Biomedical
✦ B Medical Systems
✦ Helmer Scientific
■ In April 2024, Thermo Fisher Scientific expanded its GMP storage capacity by opening a new biologics storage facility in Massachusetts to meet rising clinical trial demands.
■ Azenta Life Sciences launched a cloud-based GMP storage monitoring platform in late 2023, enhancing remote tracking and real-time compliance capabilities.
✅ Conclusion
The GMP storage market is positioned for significant growth, driven by stringent regulations, increased biologic production, and globalization of the pharmaceutical supply chain. With innovations in storage technologies and automation, companies are increasingly adopting advanced, compliant storage solutions. As the demand for precision, traceability, and safety rises, GMP storage will continue to be a cornerstone in regulated industries, ensuring product integrity from production to patient delivery. Investing in this evolving market presents a strategic advantage for stakeholders aiming for long-term success in the life sciences sector.
✅About Persistence Market Research:
At Persistence Market Research, we specialize in creating research studies that serve as strategic tools for driving business growth. Established as a proprietary firm in 2012, we have evolved into a registered company in England and Wales in 2023 under the name Persistence Research & Consultancy Services Ltd. With a solid foundation, we have completed over 3600 custom and syndicate market research projects, and delivered more than 2700 projects for other leading market research companies' clients.
Our approach combines traditional market research methods with modern tools to offer comprehensive research solutions. With a decade of experience, we pride ourselves on deriving actionable insights from data to help businesses stay ahead of the competition. Our client base spans multinational corporations, leading consulting firms, investment funds, and government departments. A significant portion of our sales comes from repeat clients, a testament to the value and trust we've built over the years.
Contact Us:
Persistence Market Research
G04 Golden Mile House, Clayponds Lane
Brentford, London, TW8 0GU UK
USA Phone: +1 646-878-6329
UK Phone: +44 203-837-5656
Email: sales@persistencemarketresearch.com
Web: https://www.persistencemarketresearch.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release GMP Storage Market Driven by Rising Demand for Regulatory Compliance and Product Safety here
News-ID: 4076844 • Views: …
More Releases from Persistence Market Research

Biosimilar Insulin Market to Reach US$ 6.0 Bn by 2033, Expanding at 14.9% CAGR | …
The biosimilar insulin market has witnessed significant growth in recent years, driven by increasing global diabetes prevalence, rising healthcare costs, and the demand for affordable alternatives to branded insulin therapies. As more people around the world develop type 1 and type 2 diabetes, the need for effective and cost-efficient insulin options has become more critical. This article provides an in-depth analysis of the biosimilar insulin market, including key growth drivers,…
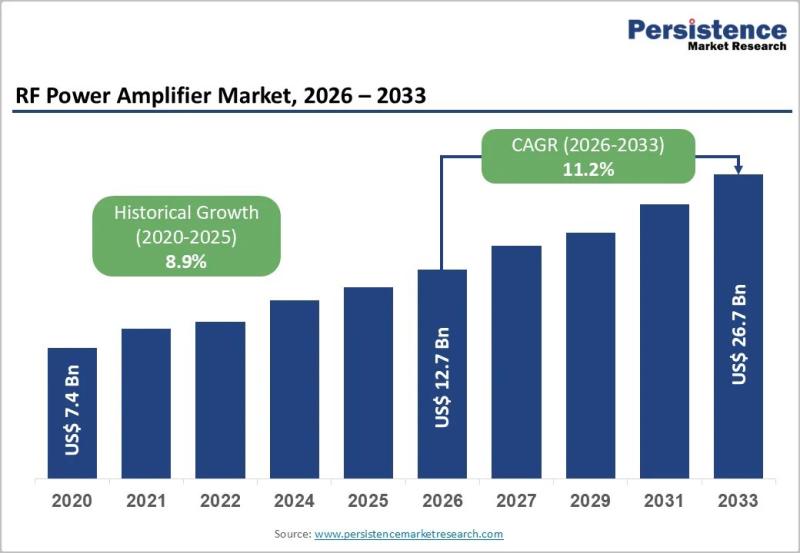
RF Power Amplifier Market Accelerates on 5G, GaN, and Defense Modernisation
RF Power Amplifier Market Overview and Growth Outlook
The global RF Power Amplifier Market is poised for sustained double-digit growth, projected to rise from US$ 12.7 billion in 2026 to US$ 26.7 billion by 2033, reflecting a strong CAGR of 11.2% during the forecast period. This expansion is underpinned by structural demand across telecommunications infrastructure, aerospace and defense modernization, and next-generation satellite communication (SATCOM) systems. As mobile data traffic surges and…
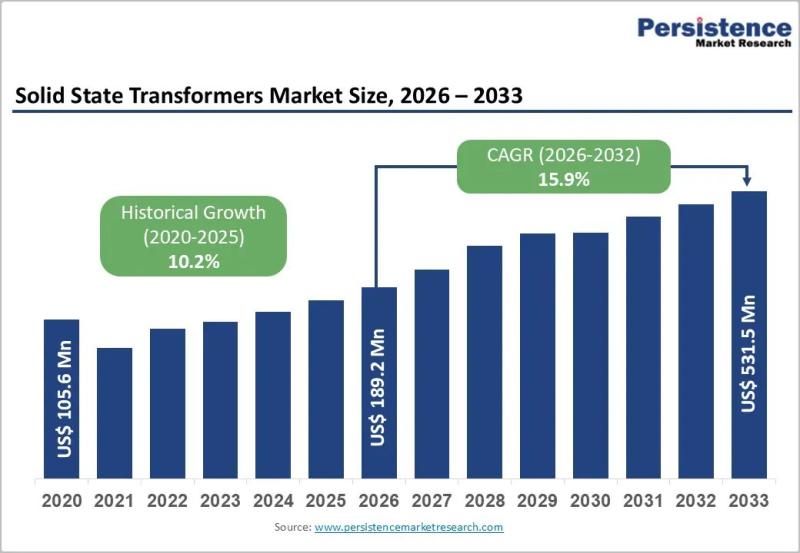
Smart Grid Expansion Driving Solid State Transformers Demand
Solid State Transformers Market Overview and Growth Outlook
The global Solid State Transformers Market is entering a high-growth phase, driven by rapid grid modernization and the accelerating shift toward renewable energy. Valued at US$ 189.2 million in 2026, the market is projected to reach US$ 531.5 million by 2033, expanding at a robust CAGR of 15.9% during the forecast period. The rising need for intelligent power distribution, bidirectional energy flow, and…
Enterprise Password Management Market to Reach US$ 9.4 Billion by 2033 as Zero-T …
The Enterprise Password Management Market is expanding rapidly as organizations confront escalating cyber threats, data breach risks, and complex regulatory mandates. Valued at US$ 3.2 billion in 2026, the market is projected to reach US$ 9.4 billion by 2033, registering a strong CAGR of 16.8% between 2026 and 2033. This accelerated growth reflects rising investment in identity security infrastructure as enterprises adopt zero-trust frameworks and strengthen credential governance.
Historically, the market…
More Releases for GMP
Creative Peptides Released GMP Synthesis Service
Located in Shirley, New York, the world’s leading peptide supplier Creative Peptides announced the launch of its GMP synthesis (https://www.creative-peptides.com/services/custom-gmp-peptide-synthesis-services.html ) business on August 29, 2018. Now this company is focused on the development and GMP manufacturing of pharmaceutical grade peptides.
As the demand of pharmaceutical market continues to grow, more and more pharmas and research institutions choose the CMO and CRO models to expand their businesses, which is more…
Diapharm implements European GMP guidelines in China
Münster (DE), London (UK), Ningbo (CN), 20 December 2013 – Pharmaceutical service provider Diapharm (diapharm.com) is increasing its business activities in China: Diapharm has now implemented a “European” quality management system for Neptune Pharma Ltd (www.neptunepharma.com) in their Joint Venture Partner’s factory in Ningbo, Zhejiang Province. And it has done so successfully: The veterinary medicinal product Trident 500mg/g Powder for Suspension for Fish Treatment (www.trident-50.com), is manufactured onsite under EU…
ECA Foundation releases free GMP WebApp
The ECA Foundation has been providing advanced training and information services in the pharmaceutical industry and especially with regard to pharmaceutical Quality Assurance and GMP compliance for more than 10 years. Now the organisation took advantage of its extensive experience to develop a further free of charge service – the new GMP WebApp.
This new GMP WebApp runs on all smartphones and tablet PCs (Apple and Android platforms) and allows users…
GMP Friction Products Awarded ISO 9001:2008
Internationally Recognized Certification Measures Consistency in Process, Procedure and Quality Performance in Manufacture of Friction Materials
AKRON, OH (March 23, 2011) -- GMP Friction Products, a world leader manufacturing powdered metal friction products for clutch plates and brake pads, recently received certification for ISO 9001:2008.
“ISO 9001:2008 signifies we have taken the extra measure of documenting the policies and standards to ensure consistent compliance with our manufacturing processes,” said Jerry Lynch,…
GMP MANUAL Volume 2 - Validation Procedures by Maas & Peither AG – GMP Publish …
GMP Publishing is launching its new GMP MANUAL Volume 2 – Validation Procedures.
The compendium on validation procedures was written by Dr. Doris Borchert, Dr. Peter Bosshard, Dr. Ralph Gomez, Dr. Michael Hiob, Dr. Christine Oechslein, Max Lazar, Ulrike Reuter, Michael Schulte, Uwe Schwarzat – all international experts and key opinion leaders. They share their detailed understanding of the various aspects of the validation process in clear and comprehensive style…
blue inspection body celebrates 50 GMP audits
Münster (Germany), 20 November 2009. Two years after founding the company and just 18 months after gaining the accreditation blue inspection body GmbH announced today the successful execution of its 50th GMP audit. Further audit trips to China, India, Israel and various European countries have been scheduled already, meaning that in the first quarter 2010 the 75th audit is targeted to be completed. Blue, as a privately organised inspection body,…
