Press release
Strong Growth Ahead: Clinical Trial Software Market Size To Grow At Arecord 14.1% Cagr By 2029
We've updated all our reports with current data on tariff changes, trade developments, and supply chain shifts affecting key industries.How Big Is the Clinical Trial Software Market Size Expected to Be by 2034?
The market size for clinical trial software has seen swift expansion in the past few years. A surge from $1.16 billion in 2024 to about $1.33 billion in 2025, represents a Compound Annual Growth Rate (CAGR) of 14.4%. The historical growth is due to a rise in demand for new software, greater embracing of innovative technologies, more digitalization in clinical trials, increased application of cloud computing, and also a growth in the number of small and mid-size biopharmaceutical firms.
The market size for clinical trial software is predicted to experience a swift expansion in the TH*Coming years. It is projected to reach $2.25 billion in 2029 with a CAGR of 14.1%. The anticipated growth within this predicted period can be ascribed to factors such as a surge in clinical research activities, the rising preference for virtual trials, increased requirement for patient-centric clinical trial solutions, growing demand for speciality medicines, and the escalating occurrence of venous diseases. Key trends during this forecast era include developments in technology, the advent of artificial intelligence (AI), progress and novelty in the technological field, the emergence of AI and machine learning, and amalgamation with real-world data.
Purchase the full report for exclusive industry analysis:
https://www.thebusinessresearchcompany.com/purchaseoptions.aspx?id=21150
What Are the Emerging Segments Within the Clinical Trial Software Market?
The clinical trial software market covered in this report is segmented -
1) By Deployment: On-Premises, Web-Based Clinical Trial Software, Cloud-Based Clinical Trial Software, Other Deployments
2) By Software: Electronic Data Capture (EDC), Electronic Clinical Outcome Assessment (eCOA) Or Electronic Patient-Reported Outcome (ePRO), Electronic Informed Consent
3) By End-User: Pharmaceutical And Biotechnology Companies, Contract Research Organizations (CROs), Medical Device Manufacturers, Other End-Users
Subsegments:
1) By On-Premises: Enterprise Clinical Trial Management Systems, Site-Based Clinical Trial Solutions
2) By Web-Based Clinical Trial Software: Electronic Data Capture (EDC) Systems, Clinical Trial Management Systems (CTMS), Randomization And Trial Supply Management (RTSM)
3) By Cloud-Based Clinical Trial Software: Software-as-a-Service (SaaS) Platforms, AI-Powered Clinical Trial Solutions, Remote Monitoring And Decentralized Trial Systems
4) By Other Deployments: Hybrid Clinical Trial Solutions, Custom-Built Clinical Trial Software
Get your free sample here:
https://www.thebusinessresearchcompany.com/sample.aspx?id=21150&type=smp
What Long-Term Drivers Are Shaping Clinical Trial Software Market Trends?
The upswing in research and development endeavors is predicted to drive the expansion of the clinical trial software market. These activities, which involve a systematic process of exploration, design, and execution of new technologies or products, aim to enhance current solutions or develop fresh offerings. The escalating demand for innovation in multiple sectors, spurred by technological advancements, competitive market dynamics, and sustainability requirements, is encouraging more investment into the creation of novel products, methods, and services. Clinical trial software assists in these pursuits by simplifying the organization, implementation, and administration of clinical trials, which are vital for the examination of new pharmaceuticals and therapies. For example, the Office for National Statistics, a government entity in the UK, reported in April 2024 that the UK government's investment into research and development surged to £15.5 ($19.67) billion in 2022 from £14.0 ($17.77) billion in the previous year, indicating a 10.5% increase. Consequently, the upturn in research and development initiatives constitutes a key driver for the enhancement of the clinical trial software market.
Who Are the Top Competitors in Key Clinical Trial Software Market Segments?
Major companies operating in the clinical trial software market are International Business Machines Corporation, Oracle Corporation, Wipro Limited, Veeva Systems, Medpace Holdings Inc., Medidata Solutions, Signant Health Inc., Clario Inc., Advarra Inc., Celerion Inc., Veristat LLC, Greenphire Inc., Medrio Inc., Arisglobal LLC, Anju Software Inc., ClinCapture Inc., Palleos TH*Care GmbH, OpenClinica LLC, Pharmaseal International Limited, SoftFormance
What Are the Major Trends Shaping the Clinical Trial Software Market?
Leading firms in the clinical trial software market are concentrating on the advancement of novel technologies, such as integrated trial solutions, to improve data compilation and consolidation, bolster patient involvement, refine trial procedures, cut down expenses, and hasten the overall drug development timeline. An Integrated Trial Solution implies a comprehensive digital platform that is developed to simplify and boost the clinical trial procedure. For instance, in November 2023, AstraZeneca plc - a pharmaceutical entity based in the UK, launched Evinova with the purpose of incorporating AI in clinical research. This revolutionary platform holds distinctive features such as automated patient enrollment, utilizing AI algorithms for quick matching of candidates with trial requirements, thereby substantially lessening enrollment periods. It comprises predictive modeling tools that scrutinize historical and real-time data to project patient reactions, amplifying the structure and effectiveness of trials. Its intuitive interface encourages improved patient participation by offering digital platforms for communication and feedback, thereby enabling participants to relay their experiences with ease.
Get the full report for exclusive industry analysis:
https://www.thebusinessresearchcompany.com/report/clinical-trial-software-global-market-report
Which Regions Are Becoming Hubs for Clinical Trial Software Market Innovation?
North America was the largest region in the clinical trial software market in 2024. Europe is expected to be the fastest-growing region in the forecast period. The regions covered in the clinical trial software market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa.
Frequently Asked Questions:
1. What Is the Market Size and Growth Rate of the Clinical Trial Software Market?
2. What is the CAGR expected in the Clinical Trial Software Market?
3. What Are the Key Innovations Transforming the Clinical Trial Software Industry?
4. Which Region Is Leading the Clinical Trial Software Market?
Why This Report Matters:
Competitive overview: This report analyzes the competitive landscape of the 3D imaging software market, evaluating key players on market share, revenue, and growth factors.
Informed Decisions: Understand key strategies related to products, segmentation, and industry trends.
Efficient Research: Quickly identify market growth, leading players, and major segments.
Connect with us on:
LinkedIn: https://in.linkedin.com/company/the-business-research-company,
Twitter: https://twitter.com/tbrc_info,
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ.
Contact Us
Europe: +44 207 1930 708,
Asia: +91 88972 63534,
Americas: +1 315 623 0293 or
Email: mailto:info@tbrc.info
Learn More About The Business Research Company
With over 15,000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Our flagship product, the Global Market Model delivers comprehensive and updated forecasts to support informed decision-making.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Strong Growth Ahead: Clinical Trial Software Market Size To Grow At Arecord 14.1% Cagr By 2029 here
News-ID: 4069306 • Views: …
More Releases from The Business Research Company
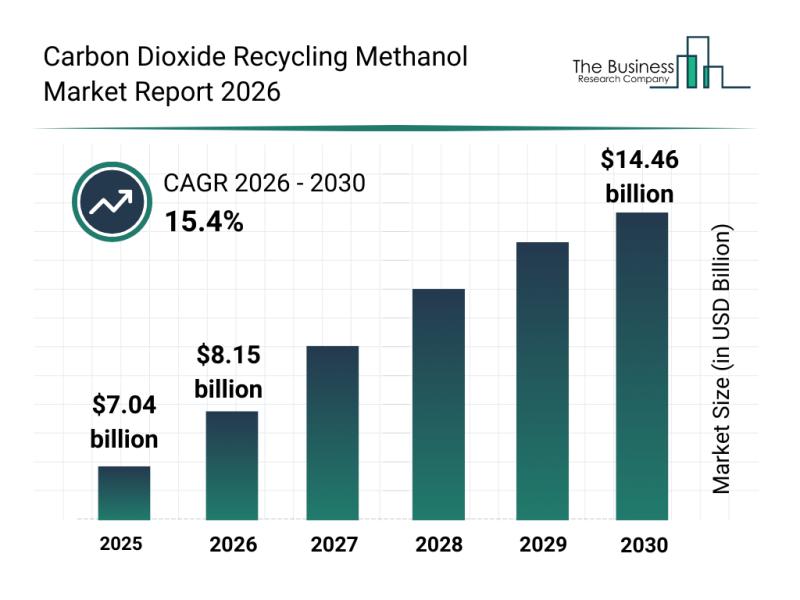
Top Players and Competitive Environment in the Carbon Dioxide Recycling Methanol …
The carbon dioxide recycling methanol market is poised for remarkable expansion as the world intensifies efforts toward sustainability and carbon neutrality. With increasing emphasis on reducing greenhouse gas emissions and boosting renewable energy sources, this market is set to play a pivotal role in the global transition to cleaner fuels and circular carbon economies. Here's a detailed look at its projected growth, influential players, emerging trends, and segmentation.
Forecasted Market Growth…
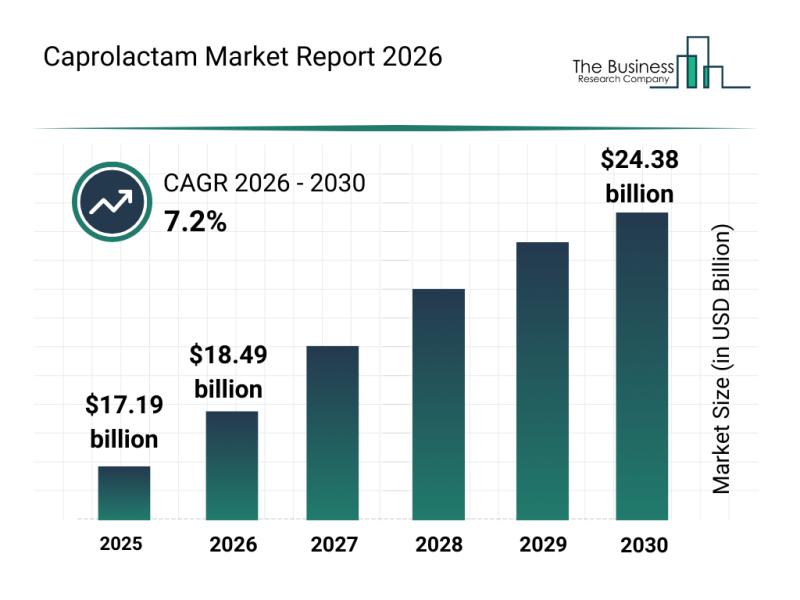
Emerging Sub-Segments Transforming the Caprolactam Market Landscape
The caprolactam market is set for significant expansion in the coming years, driven by evolving industrial needs and sustainability initiatives. This report delves into the anticipated growth, key players, emerging trends, and detailed market segmentation to offer a comprehensive view of the sector's future trajectory.
Caprolactam Market Size Forecast Through 2030
The caprolactam market is projected to reach a value of $24.38 billion by 2030, growing at a compound annual…
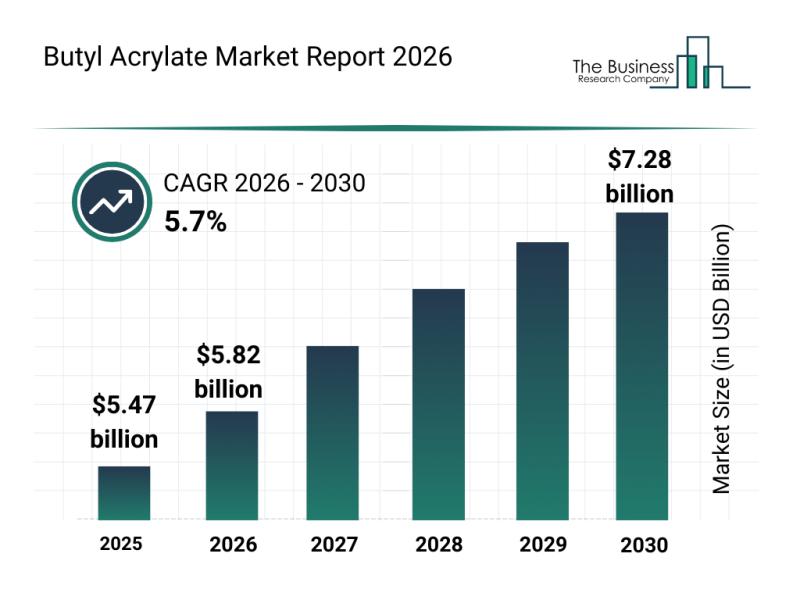
Emerging Growth Patterns Driving Expansion in the Butyl Acrylate Market
The butyl acrylate market is gaining considerable attention as industries increasingly seek versatile chemical compounds to enhance their products. With its broad utility across coatings, adhesives, and textiles, the market is set for significant growth in the coming years. Let's explore the current market size, the main players, key trends, and segment insights shaping this industry.
Projected Market Size and Growth in Butyl Acrylate
The butyl acrylate market is poised…
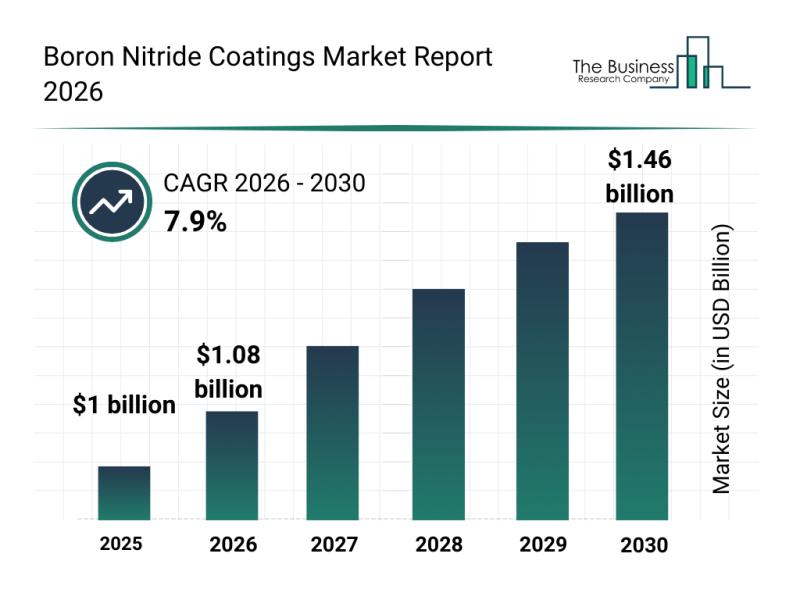
In-Depth Examination of Segments, Industry Trends, and Key Competitors in the Bo …
Boron nitride coatings are gaining increased attention due to their versatile applications and enhanced protective qualities. As industries like automotive, aerospace, and electronics evolve, the demand for advanced coating solutions that can withstand extreme conditions is rising. This overview explores the current market size, key players, important trends, and dominant segments shaping the boron nitride coatings market.
Strong Market Growth Expected for Boron Nitride Coatings by 2030
The boron nitride…
More Releases for Trial
Clinical Trial Investigative Site Network Market Clinical Trial Investigative Si …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trial Investigative Site Network Market - (By Therapeutic Areas (Oncology, Cardiology, CNS, Pain Management, Endocrine, Others), By Phase (Phase I, Phase II, Phase III, Phase IV), By End-use (Sponsor, CRO)), Trends, Industry Competition Analysis, Revenue and Forecast To 2034."
According to the latest research by InsightAce Analytic, the Global Clinical Trial Investigative Site Network Market…
Transformative Trends Impacting the Electronic Trial Master File (eTMF) Systems …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Electronic Trial Master File (eTMF) Systems Market Size By 2025?
The market size of the electronic trial master file (eTMF) systems has experienced fast growth over recent years. The market is projected to increase from $1.36 billion in 2024 to $1.55 billion in 2025, with…
Transformative Trends Impacting the Electronic Trial Master File (eTMF) Systems …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Electronic Trial Master File (eTMF) Systems Market Size By 2025?
The market size of the electronic trial master file (eTMF) systems has experienced fast growth over recent years. The market is projected to increase from $1.36 billion in 2024 to $1.55 billion in 2025, with…
Clinical Trial Imaging market
The Clinical Trial Imaging market crossed the US$ 1.09 billion mark in 2022 and is expected to hit US$ 1.94 billion by 2030, recording a CAGR of 7.5% during the forecast period.
Rising R&D spending, a rapidly growing pharmaceutical industry, and an increase in the number of contract research organizations are some of the major factors driving the market's growth. There has been an increase in pharmaceutical companies due to the…
Clinical Trial Logistics
Clinical Trial Logistics
16th to 17th May 2011, Marriott Regents Park, London, United Kingdom.
It currently costs just over £500 million ($800 million) to bring a new chemical to market and development timelines continue to fall in the 10-15 year range. A key reason for high R&D costs is due to logistical failures including failure to recruit patients on time. A way to avoid this is to move clinical trials…
Clinical Trial Logistics
Announcing SMi's 5th annual…
Clinical Trial Logistics conference
16th and 17th May 2011, Central London, UK
www.smi-online.co.uk/2011logistics-london6.asp
It currently costs just over £500 million ($800 million) to bring a new chemical to market and development timelines continue to fall in the 10-15 year range. A key reason for high R&D costs is due to logistical failures including failure to recruit patients on time. A way to avoid this is to move clinical…
