Press release
Medical Device Regulatory Affairs Market Top Players - ICON, Plc; Emergo; Freyr; Laboratory Corporation of America Holdings; IQVIA, Inc.
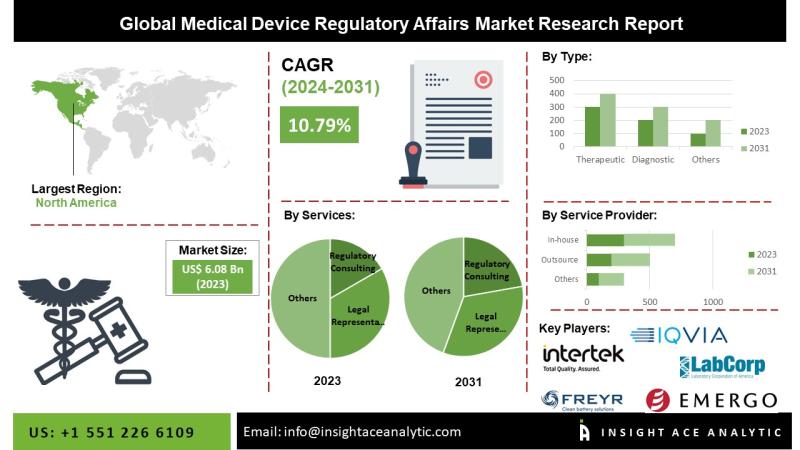
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Medical Device Regulatory Affairs Market Size, Share & Trends Analysis Report By Services (Regulatory Writing & Publishing, Legal Representation), By Type (therapeutics and diagnostics), By Service Provider (outsourced and in-house), Region, Market Outlook And Industry Analysis 2034"The global medical device regulatory affairs market is estimated to reach over USD 18.3 billion by the year 2034, exhibiting a CAGR of 10.8% during the forecast period.
Get Free Access to Demo Report, Excel Pivot and ToC: https://www.insightaceanalytic.com/request-sample/1913
Regulatory affairs (RA), also referred to as government affairs, is a specialized discipline within highly regulated industries such as pharmaceuticals, medical devices, and agrochemicals. In the healthcare sector, regulatory affairs play a critical role in ensuring compliance with evolving regulatory standards and facilitating the approval of new medical technologies.
The medical device regulatory affairs market is experiencing substantial growth, driven by several key factors. Outsourcing of regulatory services by leading market players has emerged as a strategic approach, contributing significantly to the sector's expansion. Additionally, medium- to large-sized enterprises have played a pivotal role in advancing the market, leveraging their capacity and influence to meet rising compliance demands.
This growth trajectory is largely attributed to the increasing need for expedited approval processes, continual shifts in the regulatory landscape, and the rapid expansion of emerging fields such as pharmaceuticals and diagnostics. Furthermore, government support through favorable policy initiatives and the rising complexity of medical devices are strengthening market momentum.
The global pipeline of medical devices has expanded steadily in recent years, and is expected to grow further due to heightened demand for advanced technologies and the push to enhance patient-centric designs. As a result, the demand for regulatory services is anticipated to rise in the post-pandemic era, sustaining market development and ensuring regulatory readiness for future innovations.
List of Prominent Players in the Medical Device Regulatory Affairs Market:
• ICON, Plc
• Emergo
• Freyr
• Laboratory Corporation of America Holdings
• IQVIA, Inc.
• Intertek Group plc
• SGS Société Générale de Surveillance SA
• Premedical International
• Integer Holdings Corporation
• Medpace
Expert Knowledge, Just a Click Away: https://calendly.com/insightaceanalytic/30min?month=2025-04
Market Dynamics
Drivers:
The increasing incidence of cybersecurity threats and the substantial financial implications of data breaches are compelling medical device manufacturers to implement robust security measures. This trend is reinforced by regulatory mandates and government support. Moreover, advancements in technologies such as artificial intelligence (AI), machine learning, and miniaturization are reshaping the medical device landscape, fostering demand for secure and portable devices. Additionally, strict regulatory frameworks are driving manufacturers to align with compliance standards. Key market growth factors over the forecast period include rapid technological progress, the evolution of personalized medicine, the necessity for businesses to streamline their core operations, and the intensifying competition among leading market participants.
Challenges:
Small and medium-sized enterprises (SMEs) often face significant constraints in navigating complex regulatory requirements due to limited financial and human resources. This can hinder their ability to achieve and maintain compliance, adversely affecting competitiveness. Furthermore, regulatory frameworks frequently lack standardization across regions, posing expansion challenges for globally operating companies. The dynamic nature of regulatory requirements-varying between countries and subject to ongoing updates-requires continuous monitoring and adaptation, which can be resource-intensive and operationally burdensome for international businesses.
Regional Trends:
North America is expected to hold a dominant share in the medical device regulatory affairs market, attributed to improvements in regulatory practices, cost-effectiveness, a rise in clinical trials, and the strong presence of medical device companies in the region. The availability of a skilled workforce at relatively lower costs compared to the United States further enhances the region's attractiveness. Europe also holds a significant share of the market, driven by a growing geriatric population, rising prevalence of chronic conditions, and supportive government policies aimed at strengthening the healthcare system. These factors are fueling demand for affordable medical technologies, attracting new entrants, and subsequently increasing the need for comprehensive regulatory services across the region.
Unlock Your GTM Strategy: https://www.insightaceanalytic.com/customisation/1913
Recent Developments:
• In October 2022, a Korean manufacturer of orthopedic implants entered into a partnership agreement with Freyr, which provided regulatory device registration and legal representation services.
• In August 2019-Plexus Corp. declared that their fourth facility would be certified to produce class iii finished medical devices. Class III medical registration represents the potential to produce the riskiest medical equipment, which sustains and preserves lives. This accomplishment raises the value of all types of services, such as supply chain, aftermarket, and product development.
Segmentation of Medical Device Regulatory Affairs Market-
Medical Device Regulatory Affairs Market By Services
• Regulatory Consulting
• Legal Representation
• Regulatory Writing & Publishing
• Product Registration & Clinical Trial Applications
• Other Services
Medical Device Regulatory Affairs Market By Type
• Diagnostic
• Therapeutic
Medical Device Regulatory Affairs Market By Service Provider
• Outsource
• In-house
Medical Device Regulatory Affairs Market By Region-
North America-
• The US
• Canada
• Mexico
Europe-
• Germany
• The UK
• France
• Italy
• Spain
• Rest of Europe
Asia-Pacific-
• China
• Japan
• India
• South Korea
• Southeast Asia
• Rest of Asia Pacific
Latin America-
• Brazil
• Argentina
• Rest of Latin America
Middle East & Africa-
• GCC Countries
• South Africa
• Rest of Middle East and Africa
Read Overview Report- https://www.insightaceanalytic.com/report/medical-device-regulatory-affairs-market/1913
About Us:
InsightAce Analytic is a market research and consulting firm that enables clients to make strategic decisions. Our qualitative and quantitative market intelligence solutions inform the need for market and competitive intelligence to expand businesses. We help clients gain competitive advantage by identifying untapped markets, exploring new and competing technologies, segmenting potential markets and repositioning products. Our expertise is in providing syndicated and custom market intelligence reports with an in-depth analysis with key market insights in a timely and cost-effective manner.
Contact us:
InsightAce Analytic Pvt. Ltd.
Visit: www.insightaceanalytic.com
Tel : +1 607 400-7072
Asia: +91 79 72967118
info@insightaceanalytic.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Medical Device Regulatory Affairs Market Top Players - ICON, Plc; Emergo; Freyr; Laboratory Corporation of America Holdings; IQVIA, Inc. here
News-ID: 4065833 • Views: …
More Releases from Insightace Analytic Pvt Ltd.
RNAi Pesticides Market Report on the Untapped Growth Opportunities in the Indust …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "RNAi Pesticides Market"-, By Application (Insect Pest Control, Weed Management, Disease Management, Resistance Management), By Crop Type (Agricultural Application, Non-Agricultural Application), By Product (Tropical RNAi Pesticide, Seed Embedded RANi, Transgenic RNAi, Others), By Formulation (Liquid Formulation, Granular Formulation, Powder Formulation, Others), Industry Trends, and Global Forecasts, 2025-2034 And Segment Revenue and Forecast To 2034."
The RNAi…
Rice Bran Wax Lubricant Market Know the Scope and Trends
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Rice Bran Wax Lubricant Market - (By Grade (Food Grade, Industrial Grade, Pharmaceutical Grade), End-use (Automotive, Food and Beverage, Cosmetics and Personal Care, Pharmaceuticals, Chemicals, Others)), Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
According to the latest research by InsightAce Analytic, the Global Rice Bran Wax Lubricant Market is valued at US$ 394.6 million…
Returnable Plastic Crate Market Exclusive Report with Detailed Study Analysis
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Returnable Plastic Crate Market - (By Capacity (Less than 10 kg, 10 kg to 20 kg, 21 to 35 kg, 36 to 50 kg, Above 50 kg), By Product Type (Stackable, Nestable, Collapsible), By Material (High-Density Polyethylene (HDPE), Polypropylene (P.P.), Others), By Application (Agriculture, Grocery, Dairy, Bakery, Seafood & Meat, Others)), Trends, Industry Competition…
Respiratory Disease Genetic Testing Market Exclusive Report on the Latest Revenu …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Respiratory Disease Genetic Testing Market- (By Offerings (Products (Kits and Consumables), Services, Others), By Disease Type (COPD (Alpha-1-antitrypsin (AAT), Cystic Fibrosis (CF), Diffuse Lung Disease/Surfactant Dysfunction (RHD (Respiratory Distress Syndrome), PPHN (Persistent Pulmonary Hypertension of the Newborn)), Interstitial lung disease, Pulmonary Arterial Hypertension, Pulmonary Hypoplasia, Primary Ciliary Dyskinesia, Other Diseases (BPD)), By Offerings (PCR, NGS…
More Releases for Regulatory
Medical Device Regulatory Affairs Market Medical Device Regulatory Affairs Marke …
"Medical Device Regulatory Affairs Market" in terms of revenue was estimated to be worth $ 6.7 billion in 2024 and is poised to reach $ 18.3 billion by 2034, growing at a CAGR of 10.8% from 2025 to 2034 according to a new report by InsightAce Analytic.
Request For Free Sample Pages:
https://www.insightaceanalytic.com/request-sample/1913
Latest Drivers Restraint and Opportunities Market Snapshot:
Key factors influencing the global medical device regulatory…
Medical Device & IVD Regulatory Affairs Outsourcing Market: Navigating Regulator …
Global healthcare landscape, the Medical Device & IVD Regulatory Affairs Outsourcing Market has emerged as a critical component ensuring the safe and compliant introduction of medical devices and in-vitro diagnostic products to the market. As the industry witnesses significant shifts and challenges, here's an in-depth analysis of the current trends, dynamics, and future prospects within this market segment.
Download sample PDF copy of report: https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=79264&utm_source=OpenPR_Ajay&utm_medium=OpenPR
Impact of COVID-19 on European Regulations
The outbreak of…
Regulatory Writing Market - Clear, Concise, Compliant: Redefining Regulatory Wri …
Newark, New Castle, USA - new report, titled Regulatory Writing Market The report has been put together using primary and secondary research methodologies, which offer an accurate and precise understanding of the Regulatory Writing market. Analysts have used a top-down and bottom-up approach to evaluate the segments and provide a fair assessment of their impact on the global Regulatory Writing market. The report offers an overview of the market, which…
Complex Regulatory Frameworks
It is challenging for new entrants to enter the FinTech industry because of its complex regulatory framework. All FinTech companies must comply with compliance requirements even before they begin operations, which increases their costs and creates a significant barrier for startups. While regulations are needed to protect consumers, a number of existing laws are slowing down the growth of many Indian FinTech companies, thereby extending their time to reach the…
South Africa Upstream Fiscal and Regulatory Report 2017 - Pending Legislation Cr …
Presented report, South Africa Upstream Fiscal and Regulatory Report 2017 - Pending Legislation Creates Regulatory Uncertainty, presents the essential information relating to the terms which govern investment into South Africa’s upstream oil and gas sector. The report sets out in detail the contractual framework under which firms must operate in the industry, clearly defining factors affecting profitability and quantifying the state’s take from hydrocarbon production. Considering political, economic and industry…
Regulatory Affairs Outsourcing Market (Services - Regulatory Submissions, Clinic …
This research study analyzes the market for regulatory affairs outsourcing services in terms of revenue (US$ Mn). The stakeholders of this report comprises the clinical research organizations. The global regulatory affairs outsourcing market has been broadly segmented on the basis of services (Regulatory Submissions, Clinical Trial Applications and Product Registrations, Regulatory Writing and Publishing, Regulatory Consulting and Legal Representation and others regulatory affairs, and Geography (North America, Europe, Asia Pacific,…