Press release
Sickle Cell Disease Pipeline 2025: Key Developments, Emerging Therapies, and Clinical Trials Detailed Analysis by DelveInsight | Sanofi, Novartis AG, Bluebird Biotech, Pfizer Inc., Aruvant Sciences
(Las Vegas, Nevada, United States) As per DelveInsight's assessment, globally, Sickle Cell Disease pipeline constitutes 55+ key companies continuously working towards developing 60+ Sickle Cell Disease treatment therapies, analysis of Clinical Trials, Therapies, Mechanism of Action, Route of Administration, and Developments analyzes DelveInsight.The Sickle Cell Disease Pipeline report embraces in-depth commercial and clinical assessment of the pipeline products from the pre-clinical developmental phase to the marketed phase. The report also covers a detailed description of the drug, including the mechanism of action of the drug, clinical studies, NDA approvals (if any), and product development activities comprising the technology, collaborations, mergers acquisition, funding, designations, and other product-related details.
"Sickle Cell Disease Pipeline Insight, 2025 [https://www.delveinsight.com/sample-request/sickle-cell-disease-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=gpr]" report by DelveInsight outlines comprehensive insights into the present clinical development scenario and growth prospects across the Sickle Cell Disease Market.
Some of the key takeaways from the Sickle Cell Disease Pipeline Report:
*
Companies across the globe are diligently working toward developing novel Sickle Cell Disease treatment therapies with a considerable amount of success over the years.
*
Sickle Cell Disease companies working in the treatment market are - BRL Medicine, Oryzon Genomics, GlaxoSmithKline, Agios Pharmaceuticals, Beam Therapeutics Inc., Graphite Bio, Roche, Quercis Pharma, Editas Medicin, Pfizer, Emmaus Medical, Inc, Vertex Pharmaceuticals, CRISPR therapeutics, Bluebird Bio, Pfizer, Novo Nordisk, Agios Pharmaceuticals, Alexion Pharmaceuticals, Takeda, Prolong Pharmaceuticals, Roche, Beam Therapeutics, Editas Medicine, Sangamo Therapeutics, Bellicum Pharmaceuticals, Invenux, EpiDestiny, Hillhurst Biopharmaceuticals, CSL Behring, Fulcrum Therapeutics, Sana Biotechnology, and others, are developing therapies for the Sickle Cell Disease treatment
*
Emerging Sickle Cell Disease therapies in the different phases of clinical trials are- RL 101, ORY-300, GSK 4172239D, AG-946, BEAM-101, Nula-cel, RG 6107, Isoquercetin, Renizgamglogene autogedtemcel, Inclacumab, L-glutamine, CASGEVY, CTX001, LentiGlobin BB305, Inclacumab, Etavopivat, Mitapivat, ALXN1820, TAK-755, Sanguinate, Crovalimab, BEAM101, EDIT 301, BIVV003, BPX-501, SCD-101, Nicotinamide, HBI-002, CSL889, FTX-6058, SG418, and others are expected to have a significant impact on the Sickle Cell Disease market in the coming years.
*
In January 2025, Rigel Pharmaceuticals, Inc. has announced that the first patient has been enrolled in a Phase I clinical trial investigating fostamatinib, its oral spleen tyrosine kinase (SYK) inhibitor, for the treatment of sickle cell disease (SCD). The trial, sponsored by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (NIH), aims to assess the safety and tolerability of fostamatinib in individuals affected by this chronic and debilitating disorder.
*
In January 2025, Beam Therapeutics Inc. (Nasdaq: BEAM), a biotechnology company focused on developing precision genetic medicines using base editing, has announced that it will present updated data from its BEACON Phase 1/2 clinical trial of BEAM-101 for sickle cell disease. The oral presentation will take place at the 2025 Tandem Meetings | Transplantation & Cellular Therapy Meetings, organized by the American Society for Transplantation and Cellular Therapy (ASTCT) and the Center for International Blood and Marrow Transplant Research (CIBMTR), scheduled for February 12-15, 2025, in Honolulu, Hawaii.
*
In December 2024, Beam Therapeutics Inc. (Nasdaq: BEAM), a biotechnology company specializing in precision genetic medicines using base editing, has unveiled new safety and efficacy findings from its BEACON Phase 1/2 trial of BEAM-101 in sickle cell disease (SCD) patients experiencing severe vaso-occlusive crises (VOCs). These results were highlighted in the press program at the 66th American Society of Hematology (ASH) Annual Meeting.
*
In November 2024, BioLineRx Ltd. (NASDAQ: BLRX) (TASE: BLRX), a commercial-stage biopharmaceutical company focused on developing transformative therapies in oncology and rare diseases, announced that an abstract featuring the initial results from a Phase 1 clinical trial of motixafortide, both as a monotherapy and in combination with natalizumab for CD34+ hematopoietic stem cell (HSC) mobilization for gene therapies in sickle cell disease (SCD), has been accepted for oral presentation at the 66th American Society of Hematology (ASH) Annual Meeting & Exposition, scheduled for December 7-10, 2024, in San Diego, California. This proof-of-concept study, conducted in partnership with Washington University School of Medicine in St. Louis, aims to explore alternative HSC mobilization strategies that could enhance the treatment of sickle cell disease patients pursuing gene therapy.
*
In October 2024, Zydus Lifesciences has signed a Memorandum of Agreement (MoA) with the Indian Council of Medical Research (ICMR) to begin Phase 2 clinical trials of Desidustat in patients with Sickle Cell Disease. This Phase IIa, double-blind, randomized, placebo-controlled, parallel, multi-center proof-of-concept study will be co-funded and co-monitored by INTENT, the Indian National Clinical Trial and Education Network, Clinical Studies and Trial Unit, Division of Development Research, ICMR. The study aims to assess the efficacy and safety of Desidustat oral tablets for the treatment of sickle cell disease.
*
In May 2024, BioLineRx Ltd has announced a multi-center Phase I clinical trial, sponsored by St. Jude Children's Research Hospital, Inc., to assess motixafortide for mobilizing CD34+ hematopoietic stem cells (HSCs) in the development of gene therapies for sickle cell disease (SCD).
*
In April 2024, Health Canada granted priority review to the application of the gene-editing therapy exagamglogene autotemcel (exa-cel) for patients aged 12 and older with sickle cell disease (SCD) experiencing recurrent vaso-occlusive crises (VOCs) or transfusion-dependent beta-thalassemia (TDT).
*
In January 2024, GlycoMimetics announced promising initial results from a Phase Ia clinical trial of its E-selectin antagonist, GMI-1687, as a potential treatment for sickle cell disease (SCD).
Sickle Cell Disease Overview
Sickle Cell Disease (SCD) is a genetic blood disorder caused by a mutation in the hemoglobin gene. It results in the production of abnormal hemoglobin (hemoglobin S), which causes red blood cells to become rigid and crescent-shaped. These misshapen cells can block blood flow, leading to pain, organ damage, anemia, and other complications. SCD is inherited when a child receives the defective gene from both parents. Symptoms include fatigue, pain crises, swelling, and increased risk of infections. Treatment focuses on managing symptoms, preventing complications, and may include medications, blood transfusions, or bone marrow transplants
Get a Free Sample PDF Report to know more about Sickle Cell Disease Pipeline Therapeutic Assessment-
https://www.delveinsight.com/report-store/sickle-cell-disease-pipeline-insight [https://www.delveinsight.com/report-store/sickle-cell-disease-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=gpr]
Emerging Sickle Cell Disease Drugs Under Different Phases of Clinical Development Include:
*
RL 101: BRL Medicine
*
ORY-3001: Oryzon Genomics
*
GSK 4172239D: GlaxoSmithKline
*
AG-946: Agios Pharmaceuticals
*
BEAM-101: Beam Therapeutics Inc.
*
Nula-cel: Graphite Bio
*
RG 6107: Roche
*
Isoquercetin: Quercis Pharma
*
Renizgamglogene autogedtemcel: Editas Medicin
*
Inclacumab: Pfizer
*
L-glutamine: Emmaus Medical, Inc
*
CASGEVY: Vertex Pharmaceuticals
*
ESCAPE: Beam Therapeutic
*
IHP-102: IHP Therapeutics
*
HBI-002: Hillhurst Biopharmaceuticals
*
BEAM101: Beam Therapeutics
*
EPI01: Novo Nordisk
*
VIT-2763: CSL Vifor
*
Inclacumab: Pfizer
*
L-glutamine: Emmaus Medical
*
Oxbryta: Pfizer
*
Exagamglogene autotemcel: CRISPR Therapeutics/Vertex Pharmaceuticals
*
Mitapivat: Agios Pharmaceuticals
*
Canakinumab: Novartis
*
ALXN1820: Alexion Pharmaceuticals
*
Crovalimab: Chugai Pharmaceutical/Roche
*
EDIT 301: Editas Medicine
*
BIVV003: Sangamo Therapeutics
*
BEAM101: Beam Therapeutics
*
Hemopexin: CSL Behring
Sickle Cell Disease Route of Administration
Sickle Cell Disease pipeline report provides the therapeutic assessment of the pipeline drugs by the Route of Administration. Products have been categorized under various ROAs, such as
*
Intranasal
*
Intrathecal
*
Intravenous
*
Oral
*
Oral/Intravenous
*
Parenteral
*
Subcutaneous
*
Subcutaneous/Intramuscular
*
Transdermal
Sickle Cell Disease Molecule Type
Sickle Cell Disease Products have been categorized under various Molecule types, such as
*
Antisense oligonucleotide
*
Gene therapy
*
Hormones
*
Neuropeptides
*
Oligonucleotides
*
Small Molecule
*
Triglyceride
Sickle Cell Disease Pipeline Therapeutics Assessment
*
Sickle Cell Disease Assessment by Product Type
*
Sickle Cell Disease By Stage and Product Type
*
Sickle Cell Disease Assessment by Route of Administration
*
Sickle Cell Disease By Stage and Route of Administration
*
Sickle Cell Disease Assessment by Molecule Type
*
Sickle Cell Disease by Stage and Molecule Type
DelveInsight's Sickle Cell Disease Report covers around 60+ products under different phases of clinical development like
*
Late-stage products (Phase III)
*
Mid-stage products (Phase II)
*
Early-stage product (Phase I)
*
Pre-clinical and Discovery stage candidates
*
Discontinued & Inactive candidates
*
Route of Administration
Further Sickle Cell Disease product details are provided in the report. Download the Sickle Cell Disease pipeline report to learn more about the emerging Sickle Cell Disease therapies [https://www.delveinsight.com/sample-request/sickle-cell-disease-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=gpr]
Some of the key companies in the Sickle Cell Disease Therapeutics Market include:
Key companies developing therapies for Sickle Cell Disease are - Novartis AG, Global Blood Therapeutics Inc., Emmaus Medical Inc., Addmedica, Medunik USA, Bristol Myers Squibb Co., Sanofi SA, Bluebird Biotechnology, Pfizer Inc., Aruvant Sciences Inc., Glycomimetics Inc., Editas Medicine Inc., CRISPR Therapeutic, and others.
Sickle Cell Disease Pipeline Analysis:
The Sickle Cell Disease pipeline report provides insights into
*
The report provides detailed insights about companies that are developing therapies for the treatment of Sickle Cell Disease with aggregate therapies developed by each company for the same.
*
It accesses the Different therapeutic candidates segmented into early-stage, mid-stage, and late-stage of development for Sickle Cell Disease Treatment.
*
Sickle Cell Disease key companies are involved in targeted therapeutics development with respective active and inactive (dormant or discontinued) projects.
*
Sickle Cell Disease Drugs under development based on the stage of development, route of administration, target receptor, monotherapy or combination therapy, a different mechanism of action, and molecular type.
*
Detailed analysis of collaborations (company-company collaborations and company-academia collaborations), licensing agreement and financing details for future advancement of the Sickle Cell Disease market.
The report is built using data and information traced from the researcher's proprietary databases, company/university websites, clinical trial registries, conferences, SEC filings, investor presentations, and featured press releases from company/university websites and industry-specific third-party sources, etc.
Download Sample PDF Report to know more about Sickle Cell Disease drugs and therapies [https://www.delveinsight.com/sample-request/sickle-cell-disease-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=gpr]
Sickle Cell Disease Pipeline Market Drivers
*
Increasing prevalence of SCD, rising initiatives by various governments and private organizations are some of the important factors that are fueling the Sickle Cell Disease Market.
Sickle Cell Disease Pipeline Market Barriers
*
However, clinical heterogeneity of the disease, associated complications of the disease and other factors are creating obstacles in the Sickle Cell Disease Market growth.
Scope of Sickle Cell Disease Pipeline Drug Insight
*
Coverage: Global
*
Key Sickle Cell Disease Companies: BRL Medicine, Oryzon Genomics, GlaxoSmithKline, Agios Pharmaceuticals, Beam Therapeutics Inc., Graphite Bio, Roche, Quercis Pharma, Editas Medicin, Pfizer, Emmaus Medical, Inc, Vertex Pharmaceuticals, CRISPR therapeutics, Bluebird Bio, Pfizer, Novo Nordisk, Agios Pharmaceuticals, Alexion Pharmaceuticals, Takeda, Prolong Pharmaceuticals, Roche, Beam Therapeutics, Editas Medicine, Sangamo Therapeutics, Bellicum Pharmaceuticals, Invenux, EpiDestiny, Hillhurst Biopharmaceuticals, CSL Behring, Fulcrum Therapeutics, Sana Biotechnology, and others
*
Key Sickle Cell Disease Therapies: RL 101, ORY-300, GSK 4172239D, AG-946, BEAM-101, Nula-cel, RG 6107, Isoquercetin, Renizgamglogene autogedtemcel, Inclacumab, L-glutamine, CASGEVY, CTX001, LentiGlobin BB305, Inclacumab, Etavopivat, Mitapivat, ALXN1820, TAK-755, Sanguinate, Crovalimab, BEAM101, EDIT 301, BIVV003, BPX-501, SCD-101, Nicotinamide, HBI-002, CSL889, FTX-6058, SG418, and others
*
Sickle Cell Disease Therapeutic Assessment: Sickle Cell Disease current marketed and Sickle Cell Disease emerging therapies
*
Sickle Cell Disease Market Dynamics: Sickle Cell Disease market drivers and Sickle Cell Disease market barriers
Request for Sample PDF Report for Sickle Cell Disease Pipeline Assessment and clinical trials [https://www.delveinsight.com/sample-request/sickle-cell-disease-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=gpr]
Table of Contents
1. Sickle Cell Disease Report Introduction
2. Sickle Cell Disease Executive Summary
3. Sickle Cell Disease Overview
4. Sickle Cell Disease- Analytical Perspective In-depth Commercial Assessment
5. Sickle Cell Disease Pipeline Therapeutics
6. Sickle Cell Disease Late Stage Products (Phase II/III)
7. Sickle Cell Disease Mid Stage Products (Phase II)
8. Sickle Cell Disease Early Stage Products (Phase I)
9. Sickle Cell Disease Preclinical Stage Products
10. Sickle Cell Disease Therapeutics Assessment
11. Sickle Cell Disease Inactive Products
12. Company-University Collaborations (Licensing/Partnering) Analysis
13. Sickle Cell Disease Key Companies
14. Sickle Cell Disease Key Products
15. Sickle Cell Disease Unmet Needs
16 . Sickle Cell Disease Market Drivers and Barriers
17. Sickle Cell Disease Future Perspectives and Conclusion
18. Sickle Cell Disease Analyst Views
19. Appendix
20. About DelveInsight
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports Pharma companies by providing comprehensive end-to-end solutions to improve their performance. It also offers Healthcare Consulting Services, which benefits in market analysis to accelerate business growth and overcome challenges with a practical approach.
Media Contact
Company Name: DelveInsight
Contact Person: Gaurav Bora
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=sickle-cell-disease-pipeline-2025-key-developments-emerging-therapies-and-clinical-trials-detailed-analysis-by-delveinsight-sanofi-novartis-ag-bluebird-biotech-pfizer-inc-aruvant-sciences]
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: NV
Country: United States
Website: https://www.delveinsight.com/
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Sickle Cell Disease Pipeline 2025: Key Developments, Emerging Therapies, and Clinical Trials Detailed Analysis by DelveInsight | Sanofi, Novartis AG, Bluebird Biotech, Pfizer Inc., Aruvant Sciences here
News-ID: 4062017 • Views: …
More Releases from ABNewswire

Bronx Criminal Defense Attorney David Mejia Colgan Launches New Website to Help …
BRONX, NY - Individuals facing criminal charges in New York City now have enhanced access to legal information and representation through a newly launched online resource. Bronx criminal defense attorney David Mejia Colgan, Esq. [https://dmclawny.com/] announces the launch of his firm's new website, designed to provide comprehensive information about criminal defense rights and legal options.
According to Bronx criminal defense attorney David Mejia Colgan, being charged with a crime can leave…
Enterprise E-Learning Shifts from Video Production to AI Documentation - Docsie …
AI document platform sees 300% increase in enterprise demand for video to text converter capabilities as L&D teams abandon costly video production cycles in favor of automated training documentation software
Austin, Texas - February 17, 2026 - A fundamental shift is underway in enterprise e-learning content creation. Rather than investing in new training video production, organizations are turning to AI-powered tools to extract and convert their existing video libraries into searchable,…

Step Inside a Story That Stays With You Forever: 'Sugar Grove Academy: Mother's …
'Sugar Grove Academy: Mother's Day' by Whitney Knowles is an inspiring story about finding out who you are-and learning to love that person, even when the world expects something else.
The story revolves around Cortney Gayle, an extraordinary teenager. She's a halfling-half human, half Lightning Dragon-stuck between two worlds and unsure where she belongs. Raised with human values but tied to a powerful magical legacy, Cortney's journey to understand herself is…
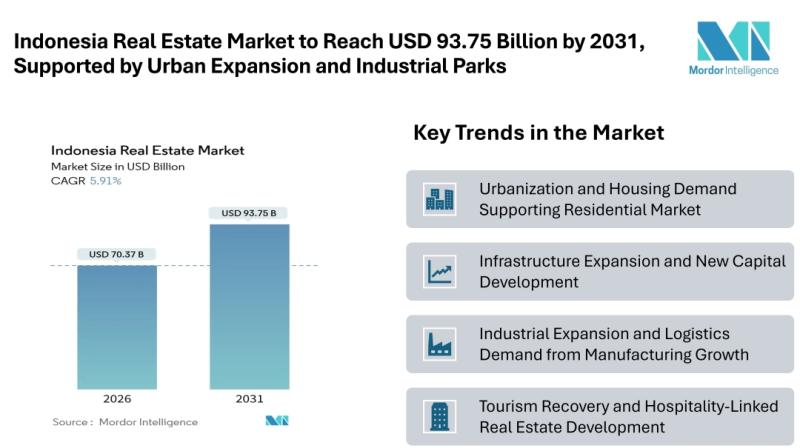
Indonesia Real Estate Market to Reach USD 93.75 Billion by 2031, Supported by Ur …
Mordor Intelligence has published a new report on the Indonesia Real Estate Market, offering a comprehensive analysis of trends, growth drivers, and future projections
Indonesia Real Estate Market Overview
According to Mordor Intelligence, the Indonesia Real Estate Market size [https://www.mordorintelligence.com/industry-reports/real-estate-market-in-indonesia?utm_source=abnewswire] is valued at USD 70.37 billion in 2026 and is projected to reach USD 93.75 billion by 2031, registering a CAGR of 5.91% during the forecast period. This steady Indonesia Real Estate…
More Releases for Cell
Cell Sorting Market Accelerates as Cell Therapy, Immuno-Oncology & Single-Cell R …
The rising focus on precision medicine, immunotherapy, and advanced cell-based research is driving the global cell sorting market into a high-growth phase. With expanding applications in stem cell therapy, CAR-T manufacturing, cancer immunology, and single-cell genomics, demand for accurate, high-purity cell isolation systems is stronger than ever. This release highlights key market trends, segmentation insights, technological innovations, and the factors shaping the future of cell sorting.
Download Full PDF Sample Copy…
Cell Isolation Cell Separation Market Size Analysis by Application, Type, and Re …
According to Market Research Intellect, the global Cell Isolation Cell Separation market under the Internet, Communication and Technology category is expected to register notable growth from 2025 to 2032. Key drivers such as advancing technologies, changing consumer behavior, and evolving market dynamics are poised to shape the trajectory of this market throughout the forecast period.
The market for cell isolation and separation is expanding rapidly as a result of sophisticated biotechnological…
Cell Free Protein Synthesis Market Beyond the Cell: Revolutionizing Protein Prod …
Cell-Free Protein Synthesis Market to reach over USD 457.13 Mn by the year 2031 - Exclusive Report by InsightAce Analytic
"Cell-Free Protein Synthesis Market" in terms of revenue was estimated to be worth $265.94 Mn in 2023 and is poised to reach $457.13 Mn by 2031, growing at a CAGR of 7.20% from 2024 to 2031 according to a new report by InsightAce Analytic.
Request for free Sample Pages: https://www.insightaceanalytic.com/request-sample/1445
Current…
Cell Expansion Market - Expand the Boundaries of Cell Therapy: Redefine Cell Exp …
Newark, New Castle, USA: The "Cell Expansion Market" provides a value chain analysis of revenue for the anticipated period from 2022 to 2030. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors
Cell Expansion Market: https://www.growthplusreports.com/report/cell-expansion-market/7939
This latest report researches the industry structure, sales, revenue,…
Global GMP Cell Banking Market By Type - Mammalian Cell, Microbial Cell, Insect …
Researchmoz added Most up-to-date research on "Global GMP Cell Banking Market By Type - Mammalian Cell, Microbial Cell, Insect Cell and Others" to its huge collection of research reports.
This report researches the worldwide GMP Cell Banking market size (value, capacity, production and consumption) in key regions like North America, Europe, Asia Pacific (China, Japan) and other regions.
This study categorizes the global GMP Cell Banking breakdown data by manufacturers, region, type…
Cell Culture Market Size, Cell Culture Market Share, Cell Culture Market Trends …
According to a new research published by Polaris Market Research the global cell culture market is anticipated to reach more than USD 49 billion by 2026. Cell culture is a rapidly emerging as an implement for analyzing and treating various disease such as Alzheimer’s and cancer.
Request for Sample of This Research Report @ https://bit.ly/2D7pZ5u
Top Key Players: -
Becton,
Dickinson and Company
Biospherix
EMD Millipore
Eppendorf AG
Merck KGaA
Sartorius AG
VWR International
Cell culture is a rapidly emerging…
