Press release
Global Biosimilar Insulin Market to Hit US$ 5.9 Bn by 2032, Growing at 14.9% CAGR: Persistence Market Research
The biosimilar insulin market has emerged as a vital segment in the global biopharmaceutical landscape, driven primarily by the increasing prevalence of diabetes and the urgent need for cost-effective treatment alternatives. Biosimilar insulins are nearly identical copies of original insulin biologics, offering comparable efficacy and safety at significantly lower prices, thus enhancing patient access worldwide.As of 2021, the global biosimilar insulin market was valued at approximately US$ 1.3 billion. According to recent industry reports, this market is projected to grow at a robust CAGR of 14.9% from 2022 to 2032, reaching a valuation of nearly US$ 5.9 billion by 2032. This sharp growth is propelled by factors such as rising diabetes cases, especially in emerging economies, increasing regulatory approvals, and a heightened focus on healthcare affordability.
Among product types, the insulin glargine biosimilars dominate, accounting for the highest revenue share due to their widespread therapeutic use and established clinical efficacy. Geographically, North America leads the market, mainly due to its advanced healthcare infrastructure, supportive regulatory frameworks like FDA's clear guidelines on biosimilars, and high adoption rates driven by policy initiatives aimed at reducing insulin costs.
The market landscape is evolving rapidly as manufacturers intensify research and development activities, innovate production techniques, and enter strategic collaborations to expand their product portfolios and global reach.
✅Get a Sample Copy of Research Report (Use Corporate Mail id for Quick Response): https://www.persistencemarketresearch.com/samples/11674
Key Highlights from the Report
➤ The global biosimilar insulin market is projected to reach US$ 5.9 billion by 2032.
➤ North America dominates the market owing to advanced healthcare infrastructure and regulatory support.
➤ The insulin glargine segment accounts for the highest revenue share among biosimilar products.
➤ Rising demand for affordable and accessible insulin therapies is fueling market expansion globally.
➤ Strategic partnerships and collaborations are shaping the competitive landscape.
➤ Innovations in biotechnological manufacturing and delivery technologies are opening new growth avenues.
How is the Biosimilar Insulin Market Segmented by Product, Application, and End-User?
The biosimilar insulin market is meticulously segmented to cater to diverse therapeutic needs and distribution channels.
By product type, the market primarily consists of biosimilar formulations such as insulin glargine, insulin lispro, insulin aspart, and insulin detemir. Among these, insulin glargine biosimilars hold the largest share, largely due to their long-acting properties and extensive clinical adoption in managing type 1 and type 2 diabetes. Emerging biosimilars of rapid-acting insulins like insulin lispro are also gaining traction as they offer improved glycemic control post meals.
By application, the market is broadly divided into treatment for type 1 diabetes and type 2 diabetes. Type 2 diabetes represents the dominant segment due to its significantly larger patient population worldwide. The growing prevalence of type 2 diabetes, especially in developing regions, drives demand for biosimilar insulin products that offer cost-effective disease management solutions. However, type 1 diabetes applications are expected to witness steady growth due to increasing diagnosis rates and improved patient awareness.
From an end-user perspective, the biosimilar insulin market serves hospitals, specialty clinics, retail pharmacies, and home care settings. Hospitals and clinics remain the primary buyers due to their large patient base and institutional procurement practices. Retail pharmacies have witnessed increased sales volumes thanks to greater accessibility and patient preference for outpatient medication. The homecare segment is growing rapidly, supported by patient education and the development of user-friendly insulin delivery devices like pens and pumps.
Which Regions Are Leading the Biosimilar Insulin Market and Why?
North America holds the leading position in the global biosimilar insulin market, accounting for over 60% of the revenue share as of 2021. This dominance is primarily attributed to a well-established healthcare system, high prevalence of diabetes, and strong regulatory support. The FDA's approval of interchangeable biosimilars like Semglee has created a favorable environment encouraging the uptake of biosimilar insulins. Additionally, government policies aimed at lowering insulin costs and increasing patient access have catalyzed market growth in this region.
Europe stands as the second-largest market, propelled by countries like the U.K., Germany, and France, where strong biopharma industries and reimbursement frameworks encourage biosimilar adoption. The European Medicines Agency (EMA) also provides clear regulatory pathways, promoting biosimilar insulin launches. Emerging economies in the Asia-Pacific region, notably India and China, are becoming critical growth hubs. India's significant investment in biotechnology and biopharmaceutical R&D, combined with a large diabetic population, accounts for its dominant 80% market share in the APAC biosimilar insulin sector. The region is expected to witness accelerated growth as affordability and accessibility improve.
Emerging markets in Latin America and the Middle East & Africa are gradually gaining traction, driven by increasing diabetes awareness and expanding healthcare infrastructure. These regions present untapped opportunities for biosimilar manufacturers aiming to extend global reach.
What Factors Are Driving Growth in the Biosimilar Insulin Market?
Market Drivers
The biosimilar insulin market is primarily driven by the escalating prevalence of diabetes globally, which has reached epidemic proportions according to WHO data. Increasing healthcare costs and the financial burden on patients have accelerated demand for affordable treatment options like biosimilars. Regulatory agencies, including the FDA and EMA, have developed clearer and more supportive pathways for biosimilar approvals, boosting market confidence.
Advancements in biotechnology have allowed manufacturers to improve biosimilar efficacy, safety, and patient compliance, fostering higher acceptance among clinicians. Moreover, growing awareness among patients and healthcare providers regarding biosimilars' equivalence to reference biologics is enhancing uptake. Strategic collaborations and partnerships between pharmaceutical companies, research institutions, and public health bodies further catalyze product development and market penetration.
Market Restraints
Despite promising growth, the biosimilar insulin market faces challenges that restrain its full potential. High manufacturing costs associated with complex biological molecules can limit affordability and production scalability. Regulatory hurdles remain significant in some regions, where ambiguous guidelines or stringent requirements delay approvals and market entry.
Another critical challenge is the lack of awareness or skepticism among patients and healthcare providers about biosimilars' efficacy and safety compared to original biologics. Additionally, logistical issues, such as cold chain storage and distribution complexities, add to operational challenges, particularly in emerging markets with underdeveloped infrastructure.
Competitive pricing strategies by originator insulin manufacturers, including aggressive discounting and patent litigations, also impact biosimilar adoption and market growth.
Market Opportunities
The biosimilar insulin market holds considerable untapped potential, especially in developing regions where diabetes prevalence is surging, but access to affordable insulin remains limited. Increased government support and public-private partnerships aimed at expanding healthcare access present growth opportunities.
There is scope for expansion into new application areas such as pediatric diabetes care and novel delivery systems including smart insulin pens and continuous glucose monitoring integrations. Innovations in bioprocessing technologies promise to lower production costs and improve supply chain efficiencies.
Collaborations between biosimilar manufacturers and healthcare providers can drive education programs to overcome skepticism and foster wider acceptance. Additionally, emerging economies in Latin America, Africa, and parts of Asia offer promising markets for biosimilar insulin expansion due to rising healthcare investments and changing disease patterns.
✅Request for Customization of the Research Report: https://www.persistencemarketresearch.com/request-customization/11674
Frequently Asked Questions about the Biosimilar Insulin Market
➤ How Big is the Biosimilar Insulin Market in 2024?
➤ Who are the Key Players in the Global Biosimilar Insulin Market?
➤ What is the Projected Growth Rate of the Biosimilar Insulin Market?
➤ What is the Market Forecast for the Biosimilar Insulin Market through 2032?
➤ Which Region is Estimated to Dominate the Biosimilar Insulin Market During the Forecast Period?
Company Insights: Leading Players in the Biosimilar Insulin Market
✦ Biocon
✦ Eli Lilly and Company
✦ Sanofi
✦ BGP Pharma
✦ Aspen
✦ Mylan (Viatris)
✦ Novo Nordisk
✦ Pfizer
Recent Developments in the Biosimilar Insulin Market
■ Biocon received FDA approval for its interchangeable biosimilar insulin glargine (Semglee®) in July 2021, marking a significant milestone for biosimilar adoption in the U.S.
■ Eli Lilly launched Rezvoglar KwikPen, a biosimilar insulin glargine product, following FDA approval in December 2021, further expanding the competitive landscape.
Conclusion
The biosimilar insulin market is poised for substantial growth over the next decade, driven by rising diabetes prevalence, increasing healthcare cost concerns, and enhanced regulatory clarity supporting biosimilar development. With an expected CAGR of nearly 15%, the market is transforming diabetes management by providing affordable, effective treatment options. North America currently leads this growth, but emerging markets, especially in Asia-Pacific and Europe, are gaining momentum.
Key market players are leveraging innovation, strategic partnerships, and regulatory approvals to broaden their biosimilar insulin portfolios. Overcoming challenges such as manufacturing costs, regulatory complexities, and awareness gaps will be crucial to unlocking the market's full potential. As biosimilar insulin becomes increasingly integrated into global healthcare frameworks, it promises to improve patient access and outcomes, making it a critical component of the future diabetes care ecosystem.
Contact Us:
Persistence Market Research
G04 Golden Mile House, Clayponds Lane
Brentford, London, TW8 0GU UK
USA Phone: +1 646-878-6329
UK Phone: +44 203-837-5656
Email: sales@persistencemarketresearch.com
Web: https://www.persistencemarketresearch.com
About Persistence Market Research:
At Persistence Market Research, we specialize in creating research studies that serve as strategic tools for driving business growth. Established as a proprietary firm in 2012, we have evolved into a registered company in England and Wales in 2023 under the name Persistence Research & Consultancy Services Ltd. With a solid foundation, we have completed over 3600 custom and syndicate market research projects, and delivered more than 2700 projects for other leading market research companies' clients.
Our approach combines traditional market research methods with modern tools to offer comprehensive research solutions. With a decade of experience, we pride ourselves on deriving actionable insights from data to help businesses stay ahead of the competition. Our client base spans multinational corporations, leading consulting firms, investment funds, and government departments. A significant portion of our sales comes from repeat clients, a testament to the value and trust we've built over the years.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Global Biosimilar Insulin Market to Hit US$ 5.9 Bn by 2032, Growing at 14.9% CAGR: Persistence Market Research here
News-ID: 4056532 • Views: …
More Releases from Persistence Market Research

Solar Cells & Modules Market to Achieve US$ 279.3 Bn by 2032, Driven by Global R …
Introduction: Solar Energy as the Backbone of the Clean Power Transition
The global energy landscape is undergoing a profound transformation as countries accelerate their shift away from fossil fuels toward cleaner and more sustainable energy sources. Among all renewable options, solar energy has emerged as one of the most scalable, cost-effective, and widely adopted solutions. Solar cells and modules form the core of this transition, enabling the conversion of sunlight into…

Cross Laminated Timber Market on Track to Reach US$3.8 Bn by 2032 Driven by Sust …
Introduction: Cross Laminated Timber as a Game Changer in Construction
The cross laminated timber market is gaining strong momentum as the global construction industry shifts toward sustainable, low-carbon building materials. Cross laminated timber, commonly known as CLT, is an engineered wood product made by layering lumber boards crosswise and bonding them together to form large, strong structural panels. This unique structure delivers high strength, dimensional stability, and excellent load-bearing capacity while…
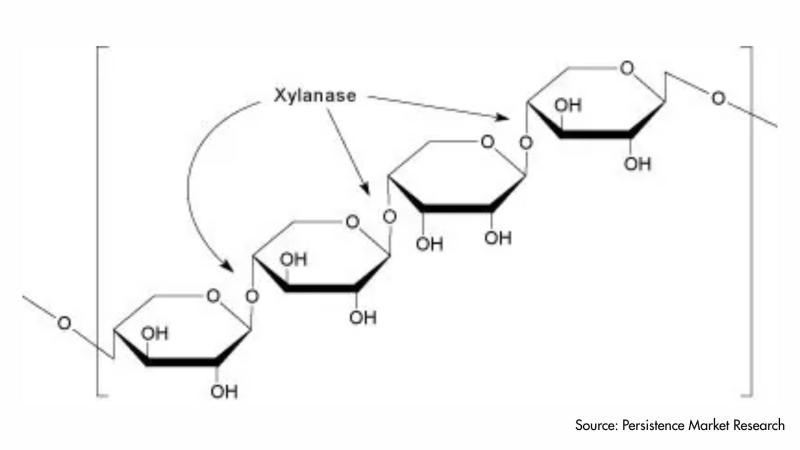
Xylanase Market US$2.7 billion by 2033 Revenue Growth Driven by Food & Feed Dema …
Introduction to the Xylanase Market Landscape
The xylanase market has emerged as a vital segment within the global industrial enzymes industry, supported by rising demand from food processing, animal nutrition, pulp and paper, and bioenergy sectors. Xylanase enzymes play a crucial role in breaking down xylan, a major hemicellulose component of plant cell walls, thereby improving processing efficiency, product quality, and sustainability outcomes. As industries increasingly prioritize cost efficiency, clean-label ingredients,…
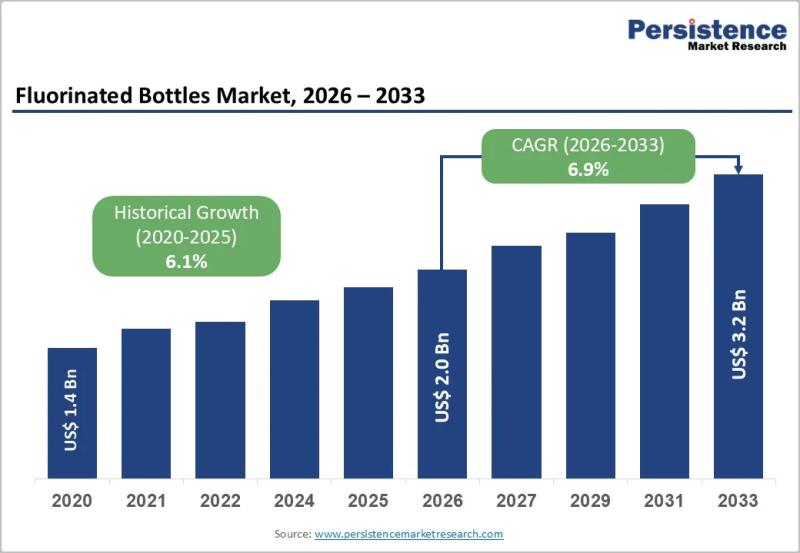
Fluorinated Bottles Market Size Valued at US$ 2.0 Billion in 2026 and Expected t …
The fluorinated bottles market has emerged as a critical segment within the rigid plastic packaging industry, addressing the growing need for safe storage and transportation of aggressive, volatile, and sensitive chemicals. Fluorinated bottles are plastic containers treated through fluorination processes to enhance their barrier properties, chemical resistance, and durability. This treatment significantly reduces permeability and prevents chemical interactions between the container and its contents, making these bottles indispensable for packaging…
More Releases for Biosimilar
Interchangeable Biosimilar Humira Market Share Driven by Biologic Therapy Adopti …
Interchangeable Biosimilar Humira Market
The global market for Interchangeable Biosimilar Humira was valued at US$ million in the year 2024 and is projected to reach a revised size of US$ million by 2031, growing at a CAGR of %during the forecast period
View sample report
https://reports.valuates.com/request/sample/QYRE-Auto-33I15005/Global_Interchangeable_Biosimilar_Humira_Market_Research_Report_2023
The Interchangeable Biosimilar Humira Market is experiencing significant market growth as healthcare providers and patients increasingly adopt biosimilar therapies for autoimmune and inflammatory conditions. Market trends indicate rising…
Key Trend Reshaping the Biosimilar Monoclonal Antibodies Market in 2025: Advance …
What Are the Projections for the Size and Growth Rate of the Biosimilar Monoclonal Antibodies Market?
In recent times, the biosimilar monoclonal antibodies sector has experienced a swift expansion. The market size, which stands at $8.04 billion in 2024, is projected to climb to $9.25 billion in 2025, marking a compound annual growth rate (CAGR) of 15.1%. Factors such as expired patents, an increased understanding of biosimilars, governmental strategies, heightened financial…
Key Trend Reshaping the Biosimilar Monoclonal Antibodies Market in 2025: Advance …
What Are the Projections for the Size and Growth Rate of the Biosimilar Monoclonal Antibodies Market?
In recent times, the biosimilar monoclonal antibodies sector has experienced a swift expansion. The market size, which stands at $8.04 billion in 2024, is projected to climb to $9.25 billion in 2025, marking a compound annual growth rate (CAGR) of 15.1%. Factors such as expired patents, an increased understanding of biosimilars, governmental strategies, heightened financial…
Biosimilar Market Treating More for Less: The Booming Infliximab Biosimilar Mark …
Infliximab Biosimilar Market worth $ XX Million by 2030 - Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Infliximab Biosimilar Market- by Application (Crohn's Disease, Psoriatic Arthritis, Rheumatoid Arthritis, Ulcerative Colitis, Ankylosing Spondylitis, Plaque Psoriasis and Others), End User (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy and Other Direct Distribution Channels), Trends, Industry Competition Analysis, Revenue and Forecast To 2030."
Get…
Biosimilar Monoclonal Antibodies Market
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the " "Global Biosimilar Monoclonal Antibodies Market by Product (infliximab, trastuzumab, rituximab, adalimumab, bevacizumab, cetuximab, ranibizumab, denosumab, eculizumab, and other pipeline products), Indication (oncology, inflammatory & autoimmune disorders, chronic diseases, blood disorders, and other indications), Clinical Trial/Pipeline Analysis, Future Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
The Biosimilar Monoclonal Antibodies Market Size is valued at 5.02…
Infliximab Biosimilar Insight, 2022 | DelveInsight
DelveInsight's, "Infliximab Biosimilar Insight, 2022" report provides comprehensive insights about 35+ companies and 45+ marketed and pipeline drugs in Infliximab Biosimilars landscape. It covers the marketed and pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Interested to know more about the functioning of…
