Press release
Clinical Trials Management System Market Key Players Analysis- Oracle; DATATRAK International, Inc.; Clario; SimpleTrials; Calyx; RealTime Software Solutions.
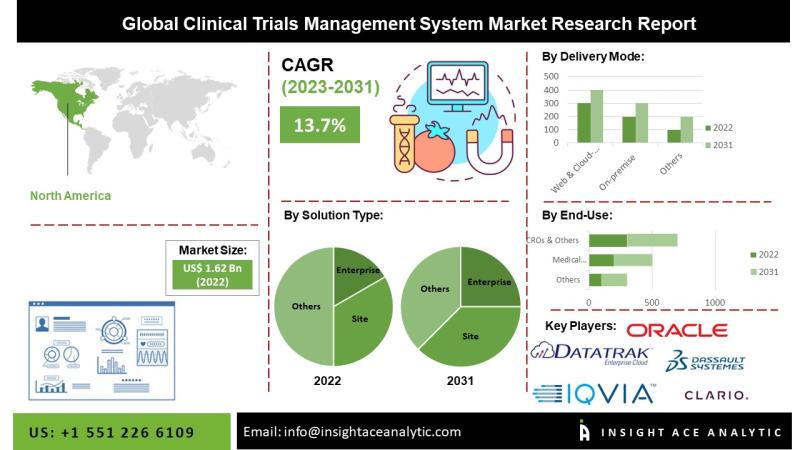
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trials Management System Market Size, Share & Trends Analysis Report By Solution Type (Enterprise and Site based), By Delivery Mode (Web & Cloud-based, On-premise), By Component (Software, Services), By End-user (Pharmaceutical and Biotechnology Firms, Medical Device Firms, CROs)- Market Outlook And Industry Analysis 2034"The global Clinical Trials Management System market is estimated to reach over 7.3 billion by the year 2034 , exhibiting a CAGR of 13.7% during the forecast period.
Get Free Access to Demo Report, Excel Pivot and ToC: https://www.insightaceanalytic.com/request-sample/1684
The growth of the clinical trial management systems (CTMS) market is predominantly driven by the increasing prevalence of chronic and lifestyle-related diseases, coupled with the expanding trend of outsourcing clinical trials to contract research organizations (CROs). As highlighted by the Alzheimer's Association in 2021, Alzheimer's dementia primarily affects individuals aged 65 and above, with prevalence rates of approximately 5.3% among those aged 65 to 74, 13.8% for those aged 75 to 84, and 34.6% for individuals aged 85 and older in the United States. The rising incidence of such chronic conditions is expected to boost demand for targeted clinical trials, thereby accelerating the adoption of CTMS solutions and fostering market expansion.
In addition, substantial investments in research and development (R&D) by pharmaceutical and biopharmaceutical companies are significantly contributing to the CTMS market's growth. According to the 2022 European Federation of Pharmaceutical Industries and Associations (EFPIA) report, pharmaceutical R&D expenditures amounted to EUR 39.7 billion in Europe, USD 72.4 billion in the United States, and CNY 78.5 billion in China in 2020. These considerable R&D investments are instrumental in driving clinical trial activities, subsequently increasing demand for CTMS platforms. Moreover, technological innovations aimed at managing escalating clinical trial costs have further supported market advancement. For example, in September 2021, Total Clinical Trial Management (TCTM) introduced the "TOTAL Diversity" brand, reflecting ongoing industry efforts to enhance clinical trial efficiency and cost-effectiveness.
List of Prominent Players in the Clinical Trials Management System Market:
• IQVIA Inc.;
• Medidata (Dassault Systèmes);
• Oracle;
• DATATRAK International, Inc.;
• Clario
• SimpleTrials;
• Calyx;
• RealTime Software Solutions, LLC;
• Laboratory Corporation of America Holdings;
• Veeva Systems; Wipro Limited;
• PHARMASEAL International Ltd
Expert Knowledge, Just a Click Away: https://calendly.com/insightaceanalytic/30min?month=2025-01
Market Dynamics
Drivers:
The clinical trial management systems (CTMS) market is projected to grow significantly, primarily due to the increasing adoption of decentralized clinical trials (DCTs). These trials, which leverage telemedicine technologies, are also referred to as virtual, digital, mobile, siteless, or remote trials. For example, Labcorp's DCT solution offers an integrated infrastructure, technological tools, and service suite aimed at optimizing the planning and execution of decentralized trials. Furthermore, the growing availability of sophisticated CTMS solutions from leading industry participants is expected to bolster market growth. A notable example is Medidata's Rave CTMS, which provides a robust automation and workflow management platform to enhance trial efficiency and oversight.
Challenges:
Despite a favorable growth trajectory, the CTMS market faces significant challenges concerning data privacy and security. The adoption of cloud-based platforms and big data technologies presents both opportunities and risks. While these tools offer potential cost advantages and operational efficiencies in managing clinical trial processes, they also necessitate stringent data protection measures to ensure compliance and mitigate cybersecurity threats.
Regional Trends:
Within North America, the United States represents the largest market for CTMS, largely attributed to high levels of research and development investment and a growing demand for innovative drug therapies. As reported by ClinicalTrials.gov, as of July 2022, there were 173,066 ongoing clinical trials in North America, with 155,448 of those located in the United States. This substantial volume of clinical activity, combined with increased funding for research initiatives, is anticipated to further stimulate CTMS market growth in the region.
Unlock Your GTM Strategy: https://www.insightaceanalytic.com/customisation/1684
Recent Developments:
• In March 2022, THREAD and Amazon Web Services agreed to work together. AWS will help develop new THREAD platform features by providing scalable automation and integrated AI to enable quicker and more effective trials by enabling higher quality data collecting throughout the clinical study lifecycle.
Segmentation of Clinical Trials Management System Market-
By Solution Type-
• Enterprise
• Site
By Delivery Mode-
• Web & Cloud-based
• On-premise
By Component-
• Software
• Services
By End User-
• Pharmaceutical and Biotechnology Firms
• Medical Device Firms
• CROs & Others
By Region-
North America-
• The US
• Canada
• Mexico
Europe-
• Germany
• The UK
• France
• Italy
• Spain
• Rest of Europe
Asia-Pacific-
• China
• Japan
• India
• South Korea
• South East Asia
• Rest of Asia Pacific
Latin America-
• Brazil
• Argentina
• Rest of Latin America
Middle East & Africa-
• GCC Countries
• South Africa
• Rest of Middle East and Africa
View Overview Report: https://www.insightaceanalytic.com/report/clinical-trials-management-system-market/1684
About Us:
InsightAce Analytic is a market research and consulting firm that enables clients to make strategic decisions. Our qualitative and quantitative market intelligence solutions inform the need for market and competitive intelligence to expand businesses. We help clients gain competitive advantage by identifying untapped markets, exploring new and competing technologies, segmenting potential markets and repositioning products. Our expertise is in providing syndicated and custom market intelligence reports with an in-depth analysis with key market insights in a timely and cost-effective manner.
Contact us:
InsightAce Analytic Pvt. Ltd.
Visit: www.insightaceanalytic.com
Tel : +1 551 226 6109
Asia: +91 79 72967118
info@insightaceanalytic.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Clinical Trials Management System Market Key Players Analysis- Oracle; DATATRAK International, Inc.; Clario; SimpleTrials; Calyx; RealTime Software Solutions. here
News-ID: 4035473 • Views: …
More Releases from Insightace Analytic Pvt Ltd.
Automotive Lead Acid Battery Market Strategic Growth Drivers and Outlook 2026 to …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Automotive Lead Acid Battery Market Size, Share & Trends Analysis Report By Product (SLI and Micro-Hybrid Batteries), Type (Flooded, Enhanced Flooded, and VRLA), Customer Segment (OEM and Aftermarket), End User (Passenger Car, Light Commercial Vehicles, Heavy Commercial Vehicles, Two-Wheeler, and Three-Wheeler), and Application (Hybrid Vehicles, Electric Vehicles, Light Motor Vehicles, and Heavy Motor Vehicles)- Market…
Automotive Interior Market Investment Opportunities and Forecast 2026 to 2035
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Automotive Interior Market- (By Component Type (Center Stack, Head-up Display, Instrument Cluster, Rear Sear Entertainment, Dome Module, Headliner, Seat, Interior Lighting Door Panel, Center Console, Adhesives & Tapes, Upholstery, Others), By Material (Leather, Fabric, Vinyl, Wood, Glass Fiber Composite, Carbon Fiber Composite, Metal), By Level of Autonomy (Semi-Autonomous, Autonomous, Non-Autonomous),By Electric Vehicle (Battery Electric Vehicle…
Artificial General Intelligence Market Future Landscape and Industry Evolution 2 …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Artificial General Intelligence (AGI) Market - (By Type of Offering (Hardware, Software and Service), Type of Technology (Machine Learning, Deep Learning, Natural Language Processing and Robotics), Mode of Deployment (Cloud-based, On Premise and Web-based), Type of AI (Weak AI, Strong AI and Superintelligence), Type of Processing (Image, Text and Voice Processing), Company Size (SMEs and…
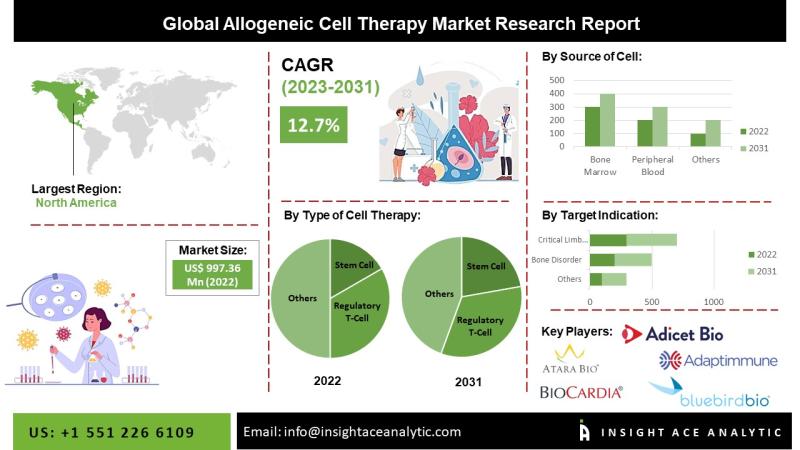
Allogenic Cell Therapies Market Revenue Trends and Growth Potential 2026 to 2035
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Allogenic Cell Therapies Market- by Cell Type(Cardiosphere-Derived Cells (CDCs), Fibroblasts, T-cells, Mesenchymal Stem Cells (MSCs), Hematopoietic Stem Cells (HSCs) and Others),Tissue Source(Skin, Blood, PBC, BM and Others), Indication (Acute graft-versus-host disease (GVHD), Chronic Ulcers and Diabetic Foot Ulcers, Osteoarthritis, Crohn's Disease, Cardiovascular Disease, Solid Tumors/Cancers and Others (Alzheimer's Disease, etc.)), Trends, Industry Competition Analysis, Revenue…
More Releases for Trial
Clinical Trial Investigative Site Network Market Clinical Trial Investigative Si …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trial Investigative Site Network Market - (By Therapeutic Areas (Oncology, Cardiology, CNS, Pain Management, Endocrine, Others), By Phase (Phase I, Phase II, Phase III, Phase IV), By End-use (Sponsor, CRO)), Trends, Industry Competition Analysis, Revenue and Forecast To 2034."
According to the latest research by InsightAce Analytic, the Global Clinical Trial Investigative Site Network Market…
Transformative Trends Impacting the Electronic Trial Master File (eTMF) Systems …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Electronic Trial Master File (eTMF) Systems Market Size By 2025?
The market size of the electronic trial master file (eTMF) systems has experienced fast growth over recent years. The market is projected to increase from $1.36 billion in 2024 to $1.55 billion in 2025, with…
Transformative Trends Impacting the Electronic Trial Master File (eTMF) Systems …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Electronic Trial Master File (eTMF) Systems Market Size By 2025?
The market size of the electronic trial master file (eTMF) systems has experienced fast growth over recent years. The market is projected to increase from $1.36 billion in 2024 to $1.55 billion in 2025, with…
Clinical Trial Management System
According to a new market report published by Persistence Market Research “Global Market Study on Clinical Trial Management System: Asia to Witness Highest Growth by 2019” the global clinical trial management system market was valued at USD 844.0 million in 2013 and is expected to grow at a CAGR of 14% from 2014 to 2019, to reach an estimated value of USD 1,848.5 million in 2019.
Request Report TOC @ https://www.persistencemarketresearch.com/methodology/3017
…
Clinical Trial Logistics
Clinical Trial Logistics
16th to 17th May 2011, Marriott Regents Park, London, United Kingdom.
It currently costs just over £500 million ($800 million) to bring a new chemical to market and development timelines continue to fall in the 10-15 year range. A key reason for high R&D costs is due to logistical failures including failure to recruit patients on time. A way to avoid this is to move clinical trials…
Clinical Trial Logistics
Announcing SMi's 5th annual…
Clinical Trial Logistics conference
16th and 17th May 2011, Central London, UK
www.smi-online.co.uk/2011logistics-london6.asp
It currently costs just over £500 million ($800 million) to bring a new chemical to market and development timelines continue to fall in the 10-15 year range. A key reason for high R&D costs is due to logistical failures including failure to recruit patients on time. A way to avoid this is to move clinical…