Press release
Investigational New Drug CDMO Market Poised for Growth with North America Leading the Way by 2031
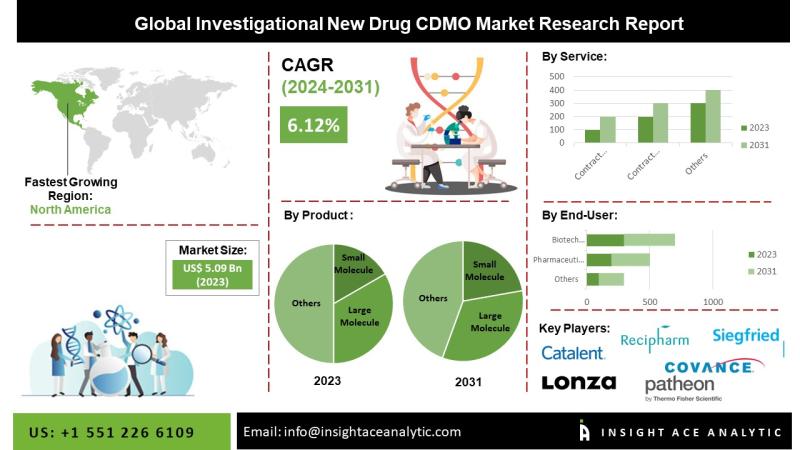
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Investigational New Drug CDMO Market By Product (Small Molecule, Large Molecule), Contract Development (Small Molecule (Bioanalysis and DMPK studies, Toxicology Testing, Pathology and safety pharmacology studies, Drug substance synthetic route development, Drug substance process development, Form selection crystallization process development, Scale-up of drug substance, Preformulation, Preclinical formulation selection, First in Man Formulation/Process Development, Analytical method development/validation, Release testing of drug substance and drug product, Work up Purification Steps, Telescoping & Process Refining, Initial Optimization, Formal stability of drug substance and drug product)), Large Molecule (Cell Line development, Process Development (Upstream (Microbial, Mammalian, Others), Downstream (MABs, Recombinant proteins, Others)), Contract Manufacturing (Small Molecule (Oral Solids, Liquid and Semi-solids, Injectables, Others), Large Molecule (MABs, Recombinant proteins, Others))), End-user (Pharmaceutical Companies, Biotech Companies, Others (Government, Research Institutes, Academic Institutes, Etc.))- Trends, Industry Competition Analysis, Revenue and Forecast To 2031."The global Investigational New Drug CDMO market is estimated to reach over 8.12 Billion by the year 2031, exhibiting a CAGR of 6.12% during the forecast period.
Get Free Access to Demo Report, Excel Pivot and ToC: https://www.insightaceanalytic.com/request-sample/1367
The increasing demand for generic medications and biologics, coupled with the capital-intensive nature of the industry and the complex manufacturing processes, has led a growing number of pharmaceutical companies to recognize the profitability of collaborating with Contract Manufacturing and Development Organizations (CMDOs) for both clinical and commercial-stage manufacturing. The expansion of this market is driven by factors such as the rising reliance on outsourcing by pharmaceutical companies, increased investments in research and development (R&D), and the implementation of stringent regulatory requirements for conducting clinical trials. The involvement of the U.S.
Food and Drug Administration (FDA) in the development of a novel drug begins when a new molecule is tested for pharmacological activity and acute toxicity in animals, and continues when the drug's sponsor seeks to investigate its diagnostic or therapeutic potential in humans. At this point, the molecule assumes a new legal status under the Federal Food, Drug, and Cosmetic Act as a novel drug, subject to the unique requirements of the drug regulatory system. While the COVID-19 pandemic significantly impacted the global economy in 2020 and continues to influence various industries, it has also presented opportunities for growth in the Investigational New Drug (IND) CDMO market. Prior to the pandemic, potential sponsors often conducted facility evaluations to ensure that CDMOs had the necessary capacity, equipment, and personnel to execute their projects. In response to the changing landscape, CDMOs have adopted innovative strategies, including the use of virtual tools such as videos and virtual reality, to engage potential sponsors and offer virtual tours of their facilities.
Expert Knowledge, Just a Click Away: https://calendly.com/insightaceanalytic/30min?month=2025-04
Market Dynamics:
Drivers:
The primary factor driving the growth of the Investigational New Drug (IND) CDMO market in the pharmaceutical industry is the increasing demand for advanced manufacturing techniques that effectively meet regulatory requirements. Pharmaceutical companies are expected to increasingly rely on outsourcing services, with rising investments in research and development (R&D) further contributing to the expansion of this market.
Challenges:
A significant challenge to the growth of the IND CDMO market is the limited availability of high investment capital. Additionally, the lack of awareness in emerging economies about the benefits of such services impedes market expansion. Furthermore, underinvestment by governments in medical infrastructure, particularly in developing countries, further constrains growth opportunities.
Regional Trends:
The North American market for IND CDMOs is projected to hold the largest market share in the near future due to its substantial patient population and favorable reimbursement policies. This is coupled with the increasing R&D expenditure by pharmaceutical and life sciences companies, which is expected to drive greater demand for contract manufacturing in the region. Market participants are adopting various strategic initiatives, such as forming new partnerships, collaborations, mergers, and acquisitions, to enhance their manufacturing capabilities and service offerings, thereby gaining a competitive advantage.
In parallel, the IND CDMO market in the Asia Pacific region is anticipated to experience rapid growth. This expansion is largely driven by the burgeoning pharmaceutical and contract manufacturing sectors in countries such as China and India. The region is seeing the rise of biotech companies, as well as a growing number of leading opinion leaders and principal investigators (PIs). Recent reforms in China aimed at improving drug review processes, accelerating the development of new medications, and reducing the time required for Investigational New Drug (IND) and New Drug Application (NDA) approvals are expected to further fuel market growth in the region.
Unlock Your GTM Strategy: https://www.insightaceanalytic.com/customisation/1367
Curious about this latest version of the report? Enquiry Before Buying:
Major market players operating in the Investigational New Drug CDMO market include Lonza, Catalent, Recipharm AB, Siegfried Holding AG, Thermo Fisher Scientific Inc., Covance Inc., Charles River Laboratories, Societal CDMO, Inc., Cambrex Corporation, FUJIFILM Diosynth Biotechnologies, Minakem, Regis Technologies Inc., Samsung Biologics, Shanghai Medicilon Inc., and TaiMed Biologics, IQVIA Holdings Inc., Syneous Health, and other prominent players.
Key developments in the market:
• In Aug 2022, Good Manufacturing Practice (GMP) accreditation has been granted to Charles River Laboratories, International Inc. Charles River's Memphis contract development and manufacturing (CDMO) facility's GMP accreditation supplements an existing GMP licence for Investigational Medicinal Product (IMP) manufacture.
• In July 2022, Lonza proposes to invest $518 million in a new fill-finish production facility in Stein, Switzerland. This project will represent the culmination of Lonza's objective to provide fully integrated CDMO services. This investment improves the company's position as a leading CDMO with an unmatched breadth of services across scales and technologies.
• In June 2021, the purchase of Vigene Biosciences, Inc. by Charles River Laboratories International, Inc. The acquisition increased its current cell and gene therapy contract manufacturing capacity and offered a comprehensive gene-modified cell treatment option in the United States.
• In Aug 2019, Permira acquired Cambrex to accelerate growth in the contract development and manufacturing organisation (CDMO) industry, which is consolidating. The investment by the Permira funds will support the continued growth of Cambrex's integrated services offering by enhancing the company's ability to service its global customer base and broadening its capabilities to provide additional world-class services to support the analysis, development, and manufacturing of drug substances and products, from preclinical through commercial phases.
Market Segments
Global Investigational New Drug CDMO Market, by Product, 2020-2030 (Value US$ Mn)
• Small Molecule
• Large Molecule
Global Investigational New Drug CDMO Market, by Service, 2020-2030 (Value US$ Mn)
• Contract Development
o Small Molecule
Bioanalysis and DMPK studies
Toxicology Testing
Pathology and safety pharmacology studies
Drug substance synthetic route development
Drug substance process development
Form selection crystallization process development
Scale-up of drug substance
Preformulation
Preclinical formulation selection
First in Man Formulation/Process Development
Analytical method development/validation
Release testing of drug substance and drug product
Work up Purification Steps
Telescoping & Process Refining
Initial Optimization
Formal stability of drug substance and drug product
o Large Molecule
Cell Line development
Process Development
• Upstream
o Microbial
o Mammalian
o Others
• Downstream
o MABs
o Recombinant proteins
o Others
• Contract Manufacturing
o Small Molecule
Oral Solids
Liquid and Semi-solids
Injectables
Others
o Large Molecule
MABs
Recombinant proteins
Others
Global Investigational New Drug CDMO Market, by End-Users, 2020-2030 (Value US$ Mn)
• Hospitals & Surgical Centers
• Ambulatory Care Centers
• Research Laboratories & Academic Institutes
Global Investigational New Drug CDMO Market, by Region, 2020-2030 (Value US$ Mn)
• North America
• Europe
• Asia Pacific
• Latin America
• Middle East & Africa
North America Investigational New Drug CDMO Market, by Country, 2020-2030 (Value US$ Mn)
• U.S.
• Canada
Europe Investigational New Drug CDMO Market, by Country, 2020-2030 (Value US$ Mn)
• Germany
• France
• Italy
• Spain
• Russia
• Rest of Europe
Asia Pacific Investigational New Drug CDMO Market, by Country, 2020-2030 (Value US$ Mn)
• India
• China
• Japan
• South Korea
• Australia & New Zealand
Latin America Investigational New Drug CDMO Market, by Country, 2020-2030 (Value US$ Mn)
• Brazil
• Mexico
• Rest of Latin America
Middle East & Africa Investigational New Drug CDMO Market, by Country, 2020-2030 (Value US$ Mn)
• GCC Countries
• South Africa
• Rest of Middle East & Africa
Why should buy this report:
To receive a comprehensive analysis of the prospects for the global Investigational New Drug CDMO market
To receive an industry overview and future trends of the Investigational New Drug CDMO market
To analyze the Investigational New Drug CDMO market drivers and challenges
To get information on the Investigational New Drug CDMO market size (Value US$ Mn) forecast to 2030
Major investments, mergers & acquisitions in the Investigational New Drug CDMO market industry
Read Overview Report- https://www.insightaceanalytic.com/report/global-investigational-new-drug-cdmo-market/1367
About Us:
InsightAce Analytic is a market research and consulting firm that enables clients to make strategic decisions. Our qualitative and quantitative market intelligence solutions inform the need for market and competitive intelligence to expand businesses. We help clients gain competitive advantage by identifying untapped markets, exploring new and competing technologies, segmenting potential markets and repositioning products. Our expertise is in providing syndicated and custom market intelligence reports with an in-depth analysis with key market insights in a timely and cost-effective manner.
Contact us:
InsightAce Analytic Pvt. Ltd.
Visit: www.insightaceanalytic.com
Tel : +1 551 226 6109
Asia: +91 79 72967118
info@insightaceanalytic.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Investigational New Drug CDMO Market Poised for Growth with North America Leading the Way by 2031 here
News-ID: 4012800 • Views: …
More Releases from Insightace Analytic Pvt Ltd.
Automotive Lead Acid Battery Market Strategic Growth Drivers and Outlook 2026 to …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Automotive Lead Acid Battery Market Size, Share & Trends Analysis Report By Product (SLI and Micro-Hybrid Batteries), Type (Flooded, Enhanced Flooded, and VRLA), Customer Segment (OEM and Aftermarket), End User (Passenger Car, Light Commercial Vehicles, Heavy Commercial Vehicles, Two-Wheeler, and Three-Wheeler), and Application (Hybrid Vehicles, Electric Vehicles, Light Motor Vehicles, and Heavy Motor Vehicles)- Market…
Automotive Interior Market Investment Opportunities and Forecast 2026 to 2035
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Automotive Interior Market- (By Component Type (Center Stack, Head-up Display, Instrument Cluster, Rear Sear Entertainment, Dome Module, Headliner, Seat, Interior Lighting Door Panel, Center Console, Adhesives & Tapes, Upholstery, Others), By Material (Leather, Fabric, Vinyl, Wood, Glass Fiber Composite, Carbon Fiber Composite, Metal), By Level of Autonomy (Semi-Autonomous, Autonomous, Non-Autonomous),By Electric Vehicle (Battery Electric Vehicle…
Artificial General Intelligence Market Future Landscape and Industry Evolution 2 …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Artificial General Intelligence (AGI) Market - (By Type of Offering (Hardware, Software and Service), Type of Technology (Machine Learning, Deep Learning, Natural Language Processing and Robotics), Mode of Deployment (Cloud-based, On Premise and Web-based), Type of AI (Weak AI, Strong AI and Superintelligence), Type of Processing (Image, Text and Voice Processing), Company Size (SMEs and…
Allogenic Cell Therapies Market Revenue Trends and Growth Potential 2026 to 2035
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Allogenic Cell Therapies Market- by Cell Type(Cardiosphere-Derived Cells (CDCs), Fibroblasts, T-cells, Mesenchymal Stem Cells (MSCs), Hematopoietic Stem Cells (HSCs) and Others),Tissue Source(Skin, Blood, PBC, BM and Others), Indication (Acute graft-versus-host disease (GVHD), Chronic Ulcers and Diabetic Foot Ulcers, Osteoarthritis, Crohn's Disease, Cardiovascular Disease, Solid Tumors/Cancers and Others (Alzheimer's Disease, etc.)), Trends, Industry Competition Analysis, Revenue…
More Releases for CDMO
FDP CDMO Research: China FDP CDMO market size is projected to reach USD 1.33 bil …
QY Research Inc. (Global Market Report Research Publisher) announces the release of 2025 latest report "Fraud Detection and Prevention (FDP) System- Global Market Share and Ranking, Overall Sales and Demand Forecast 2025-2031". Based on current situation and impact historical analysis (2020-2024) and forecast calculations (2025-2031), this report provides a comprehensive analysis of the global Wire Drawing Dies market, including market size, share, demand, industry development status, and forecasts for the…
Global Cmo And Cdmo Biotechnology Market Size by Application, Type, and Geograph …
According to Market Research Intellect, the global Cmo And Cdmo Biotechnology market under the Internet, Communication and Technology category is expected to register notable growth from 2025 to 2032. Key drivers such as advancing technologies, changing consumer behavior, and evolving market dynamics are poised to shape the trajectory of this market throughout the forecast period.
Biologics and sophisticated medicines are driving the biotechnology industry for Contract Manufacturing Organizations (CMO) and Contract…
Evolving Market Trends In The Inhalation CDMO Industry: Strategic Collaborations …
The Inhalation CDMO Market Report by The Business Research Company delivers a detailed market assessment, covering size projections from 2025 to 2034. This report explores crucial market trends, major drivers and market segmentation by [key segment categories].
What Is the Expected Inhalation CDMO Market Size During the Forecast Period?
In recent times, the inhalation CDMO market has experienced significant growth. The market value is expected to increase from $2.08 billion in 2024…
What's Driving the Inhalation CDMO Market 2025-2034: Rising Respiratory Disorder …
How Is the Chondroplasty Market Projected to Grow, and What Is Its Market Size?
The chondroplasty market has seen strong growth in recent years. It will increase from $13.77 billion in 2024 to $14.68 billion in 2025 at a CAGR of 6.5%. This growth is attributed to the rise in sports-related injuries, patient preference for non-total joint replacement procedures, advances in postoperative care, healthcare provider training, and an increasing incidence of…
Lentiviral Vector (LVV) CDMO Services Market Delivering Cures: The Role of LVV C …
Lentiviral Vector (LVV) CDMO Services Market to Record an Exponential CAGR by 2031 - Exclusive Report by InsightAce Analytic Pvt. Ltd.
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Lentiviral Vector (LVV) CDMO Services Market - (By Type (IIT Grade, IND Grade, Clinical Trial Grade, Commercial Production Grade), By Application (Biopharmaceutical Company, Academic Scientific Research Institution)), Trends, Industry Competition Analysis, Revenue and Forecast To…
Electronic Chemicals CDMO Market Fueling the Electronics Boom: The Rise of the E …
Electronic Chemicals CDMO Market to Record an Exponential CAGR by 2031 - Exclusive Report by InsightAce Analytic Pvt. Ltd.
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Electronic Chemicals CDMO Market - (By Type (Metals and Pastes, Electronic Specialty Gases, Polymer Compounds, Others), By Application (Battery, Semiconductor, Integrated Circuit, Consumer Electronics, Others)), Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
According to the latest…