Press release
GMP Testing Service Market Anticipated to Grow Significantly by 2031 - Persistence Market Research
Overview of the GMP Testing Service MarketThe GMP Testing Service Market is gaining significant traction as industries such as pharmaceuticals, biotechnology, cosmetics, and food & beverages emphasize compliance with Good Manufacturing Practices (GMP). As companies aim to meet stringent regulatory guidelines and ensure consumer safety, demand for third-party GMP testing has surged. According to Persistence Market Research, the global GMP Testing Service Market is projected to grow at a robust CAGR during the forecast period, driven by increasing regulatory scrutiny and globalization of pharmaceutical manufacturing.
In terms of market segments, the pharmaceutical industry remains the dominant end-user, owing to strict quality mandates enforced by regulatory agencies like the FDA and EMA. North America stands out as the leading region in this space, thanks to advanced regulatory frameworks, a strong pharmaceutical presence, and high awareness regarding quality control measures. This leadership is likely to persist due to the region's continued investments in healthcare infrastructure and innovation.
Get a Sample PDF Brochure of the Report (Use Corporate Email ID for a Quick Response): https://www.persistencemarketresearch.com/samples/33233
Key Highlights from the Report
• The GMP Testing Service Market is poised for steady growth, with increasing outsourcing trends in the pharmaceutical sector.
• North America remains the top revenue-generating region due to regulatory mandates and pharmaceutical R&D activities.
• The pharmaceutical industry continues to be the dominant end-user of GMP testing services.
• Outsourced testing services are in high demand due to cost-efficiency and scalability benefits.
• Stringent global regulatory requirements are compelling manufacturers to prioritize GMP testing.
• Advanced analytical techniques are increasingly being adopted to improve testing precision and compliance assurance.
Market Segmentation
The GMP Testing Service Market is segmented based on product type, including microbiological testing, chemical testing, physical characterization, and stability testing. Microbiological testing leads the market, driven by the critical need to detect microbial contamination in pharmaceuticals and biologics. Chemical testing also plays a vital role in ensuring ingredient integrity and conformity to specifications.
From an end-user perspective, pharmaceutical and biotechnology companies are the largest consumers of GMP testing services. These firms rely heavily on third-party testing to ensure compliance, especially during clinical development and product launches. Other significant users include cosmetic and nutraceutical companies, where product safety and efficacy are crucial for regulatory approval and consumer trust.
Regional Insights
In North America, the GMP testing services market has matured due to strong regulatory institutions such as the FDA. The region boasts a high concentration of pharmaceutical and biotech firms that regularly require compliance services, thereby contributing to consistent demand.
Asia Pacific is emerging as a fast-growing region in this market. Countries like China and India are expanding their pharmaceutical manufacturing bases, leading to increased outsourcing of GMP testing services. The region is also witnessing growing awareness and enforcement of GMP standards, further driving market expansion.
Market Drivers
The primary driver behind the GMP Testing Service Market is the increasing complexity of pharmaceutical and biologic products, which necessitates rigorous quality assurance processes. Global regulatory bodies are tightening compliance requirements, compelling companies to conduct regular and comprehensive testing to avoid penalties and ensure public safety. Moreover, the rise in contract manufacturing has created additional demand for independent testing services.
Market Restraints
Despite its promising outlook, the market faces several restraints. High costs associated with advanced GMP testing and validation processes may limit adoption among small to medium-sized enterprises. Additionally, variability in regulatory standards across different regions can make compliance more challenging for global firms, reducing the efficiency of testing service providers.
Market Opportunities
Opportunities are abundant in this market, especially with the rising trend of personalized medicine and biologics, which require sophisticated and customized testing protocols. The increasing demand for biosimilars and generic drugs is also opening avenues for GMP testing service providers. Technological advancements such as automation, AI-based analytics, and cloud-based LIMS (Laboratory Information Management Systems) are expected to streamline processes and attract more companies to outsource their GMP testing needs.
Reasons to Buy the Report
✔ Gain insights into the global GMP Testing Service Market and its growth potential through 2032.
✔ Understand regional dynamics and key segments that offer lucrative investment opportunities.
✔ Identify the top players and their competitive strategies to strengthen market positioning.
✔ Leverage detailed forecasts and trend analysis for strategic decision-making.
✔ Access comprehensive data on market drivers, restraints, and future opportunities.
Company Insights
Key Players in the GMP Testing Service Market:
1. Eurofins Scientific
2. SGS SA
3. Charles River Laboratories
4. Thermo Fisher Scientific Inc.
5. Merck KGaA
6. WuXi AppTec
7. Intertek Group plc
8. Sartorius AG
9. BioReliance Corporation
10. Pace Analytical Services LLC
Recent Developments:
• In 2024, Eurofins Scientific expanded its microbiological and stability testing capabilities in its U.S. laboratories to meet growing demand from pharmaceutical clients.
• SGS SA launched a cloud-based compliance dashboard in 2023 to improve real-time access to GMP testing reports for its clients worldwide.
Conclusion
The GMP Testing Service Market is evolving rapidly in response to global regulatory changes, increasing pharmaceutical output, and rising demand for product safety and efficacy. As companies navigate stringent compliance landscapes, the need for specialized testing services will continue to grow. Outsourcing to certified laboratories is not just a strategic choice-it is a regulatory necessity in today's highly regulated industries.
Persistence Market Research notes that the market's long-term trajectory remains optimistic, bolstered by innovations in analytical testing and a rising preference for third-party validation. As testing requirements become more complex and critical for market entry, the role of GMP testing services will remain indispensable across the pharmaceutical, biotech, and food sectors. Industry players who invest in advanced testing technologies, expand regional presence, and foster regulatory collaborations will be well-positioned to capitalize on this dynamic and growing market.
About Persistence Market Research:
At Persistence Market Research, we specialize in creating research studies that serve as strategic tools for driving business growth. Established as a proprietary firm in 2012, we have evolved into a registered company in England and Wales in 2023 under the name Persistence Research & Consultancy Services Ltd. With a solid foundation, we have completed over 3600 custom and syndicate market research projects, and delivered more than 2700 projects for other leading market research companies' clients.
Our approach combines traditional market research methods with modern tools to offer comprehensive research solutions. With a decade of experience, we pride ourselves on deriving actionable insights from data to help businesses stay ahead of the competition. Our client base spans multinational corporations, leading consulting firms, investment funds, and government departments. A significant portion of our sales comes from repeat clients, a testament to the value and trust we've built over the years.
Contact Us:
Persistence Market Research
G04 Golden Mile House, Clayponds Lane
Brentford, London, TW8 0GU UK
USA Phone: +1 646-878-6329
UK Phone: +44 203-837-5656
Email: sales@persistencemarketresearch.com
Web: https://www.persistencemarketresearch.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release GMP Testing Service Market Anticipated to Grow Significantly by 2031 - Persistence Market Research here
News-ID: 4012223 • Views: …
More Releases from Persistence Market Research

Cryogenic Storage Tanks Market Predicted to Hit US$ 12.8 Billion by 2033 Driven …
According to the latest study by Persistence Market Research, the global cryogenic storage tanks market is likely to be valued at US$ 8.6 billion in 2026 and is projected to reach US$ 12.8 billion by 2033, expanding at a CAGR of 5.8% during the forecast period 2026-2033. Rising demand for liquefied gases across energy, healthcare, food processing, and industrial manufacturing sectors is emerging as a key driver shaping the market's…
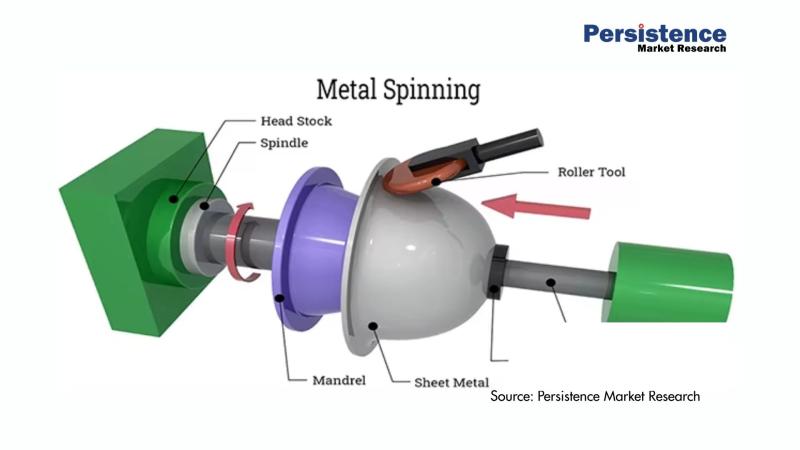
Metal Spinning Products Market Projected to Grow to US$ 4.0 billion by 2033 - Pe …
The global metal spinning products market is poised for substantial growth in the coming years. According to a recent study by Persistence Market Research, the market size is anticipated to reach US$ 4.0 billion by 2033, growing at a robust compound annual growth rate (CAGR) of 4.2% from its current valuation of US$ 3.0 billion in 2026. Metal spinning, a process of shaping metal into precise and symmetrical shapes, is…
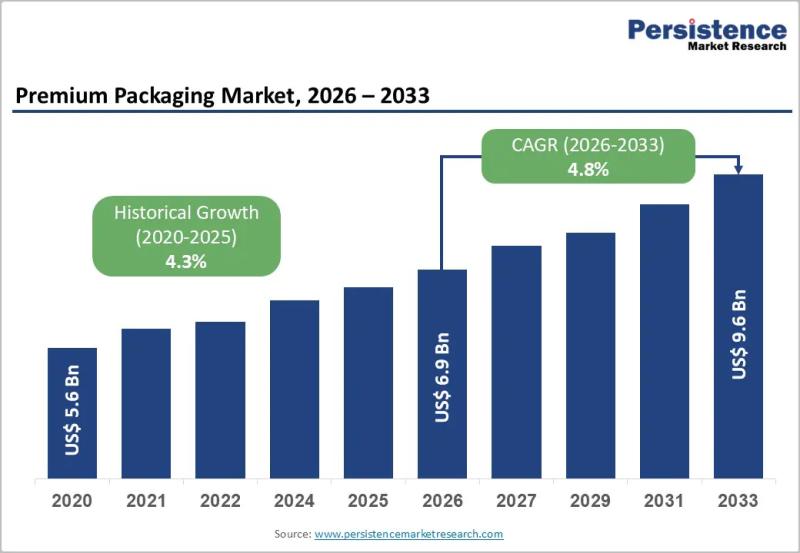
Premium Packaging Market Size Worth US$9.6 Billion by 2033 - Persistence Market …
The premium packaging market has evolved into a critical strategic element for brand differentiation across multiple high value consumer industries. Premium packaging goes beyond basic containment and protection to deliver enhanced aesthetics tactile appeal storytelling and emotional connection. Brands increasingly view packaging as an extension of their identity and a powerful marketing tool that influences purchasing decisions at the point of sale and during the unboxing experience. This shift is…
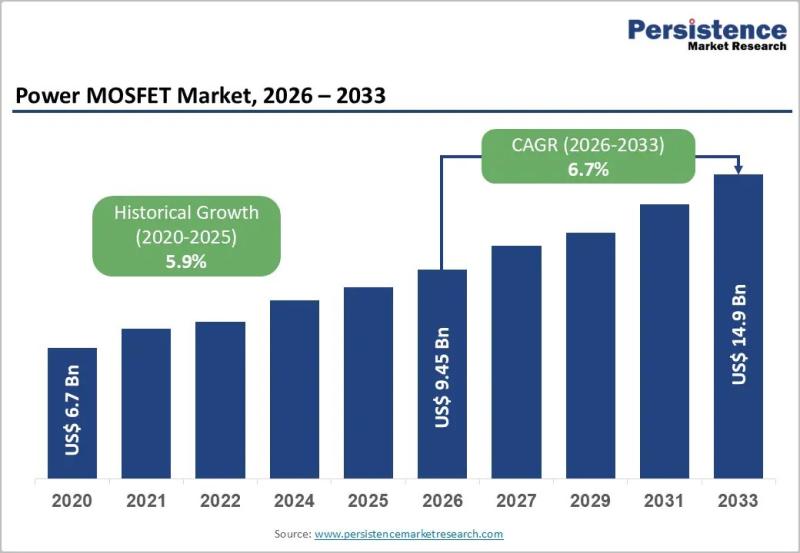
Power MOSFET Market Growth Driven by EVs Renewable Energy and Smart Automation
The global Power MOSFET market is entering a phase of sustained expansion, driven by the accelerating need for energy-efficient and high-performance power management components across industries. In 2026, the market is expected to be valued at US$ 9.45 billion and is forecast to reach US$ 14.9 billion by 2033, registering a healthy CAGR of 6.7% during the forecast period. Power MOSFETs are essential semiconductor devices that enable efficient switching and…
More Releases for GMP
Creative Peptides Released GMP Synthesis Service
Located in Shirley, New York, the world’s leading peptide supplier Creative Peptides announced the launch of its GMP synthesis (https://www.creative-peptides.com/services/custom-gmp-peptide-synthesis-services.html ) business on August 29, 2018. Now this company is focused on the development and GMP manufacturing of pharmaceutical grade peptides.
As the demand of pharmaceutical market continues to grow, more and more pharmas and research institutions choose the CMO and CRO models to expand their businesses, which is more…
Diapharm implements European GMP guidelines in China
Münster (DE), London (UK), Ningbo (CN), 20 December 2013 – Pharmaceutical service provider Diapharm (diapharm.com) is increasing its business activities in China: Diapharm has now implemented a “European” quality management system for Neptune Pharma Ltd (www.neptunepharma.com) in their Joint Venture Partner’s factory in Ningbo, Zhejiang Province. And it has done so successfully: The veterinary medicinal product Trident 500mg/g Powder for Suspension for Fish Treatment (www.trident-50.com), is manufactured onsite under EU…
ECA Foundation releases free GMP WebApp
The ECA Foundation has been providing advanced training and information services in the pharmaceutical industry and especially with regard to pharmaceutical Quality Assurance and GMP compliance for more than 10 years. Now the organisation took advantage of its extensive experience to develop a further free of charge service – the new GMP WebApp.
This new GMP WebApp runs on all smartphones and tablet PCs (Apple and Android platforms) and allows users…
GMP Friction Products Awarded ISO 9001:2008
Internationally Recognized Certification Measures Consistency in Process, Procedure and Quality Performance in Manufacture of Friction Materials
AKRON, OH (March 23, 2011) -- GMP Friction Products, a world leader manufacturing powdered metal friction products for clutch plates and brake pads, recently received certification for ISO 9001:2008.
“ISO 9001:2008 signifies we have taken the extra measure of documenting the policies and standards to ensure consistent compliance with our manufacturing processes,” said Jerry Lynch,…
GMP MANUAL Volume 2 - Validation Procedures by Maas & Peither AG – GMP Publish …
GMP Publishing is launching its new GMP MANUAL Volume 2 – Validation Procedures.
The compendium on validation procedures was written by Dr. Doris Borchert, Dr. Peter Bosshard, Dr. Ralph Gomez, Dr. Michael Hiob, Dr. Christine Oechslein, Max Lazar, Ulrike Reuter, Michael Schulte, Uwe Schwarzat – all international experts and key opinion leaders. They share their detailed understanding of the various aspects of the validation process in clear and comprehensive style…
blue inspection body celebrates 50 GMP audits
Münster (Germany), 20 November 2009. Two years after founding the company and just 18 months after gaining the accreditation blue inspection body GmbH announced today the successful execution of its 50th GMP audit. Further audit trips to China, India, Israel and various European countries have been scheduled already, meaning that in the first quarter 2010 the 75th audit is targeted to be completed. Blue, as a privately organised inspection body,…
