Press release
Insulin Biosimilars Market Value Share, Supply Demand, share and Value Chain 2016-2026
A new race has been initiated between biosimilar and branded drug manufacturers to gain maximum revenue share from insulin market. The upcoming three years are very crucial for insulin manufacturers, as most of the branded insulin are going to be off patent which would results into the loss of around US$53 Bn revenue for branded manufacturers in 2016. Generic manufacturers are expected to take smaller chunk of this amount owing to their local presence which would restrain them take share from other markets. In such dynamic situation, the original drug manufacturers are seeking for plan B to sustain their hold in the market. As a result, these companies are now integrated with biosimilar manufacturers through agreement, alliance. Moreover, legal troubles pertaining patent infringement is hampering the development insulin Biosimilars. In order to overcome such infringement issues, the companies are now collaborating with each other to develop biosimilars versions of insulin. Recent example of global collaboration agreement between Mylan N.V. and Momenta Pharmaceuticals, Inc. has provided a competitive advantage to Mylan by increasing the number of insulin biosimlars under development. This collaboration will capitalized Mylan’s existing portfolio of biologics and insulin analog development.Insulin Biosimilars Market: Drivers and Restraints
Increasing prevalence of Type I diabetes, higher cost of existing insulin drugs are expected to drive growth of insulin biosimilars market. Government authorities are also focusing on the approval of insulin biosimilars owing to substantial financial burden in terms of reimbursements. Recently, the U.S. FDA has approved new insulin glargine Basaglar, for type 1 and type 2 diabetes which is Biosimilar version of Sanofi’s basal insulin Lantus (insulin glargine). Additionally, Lilly and Boehringer Ingelheim's biosimilar insulin glargine has got approval through European Medicines Agency's (EMA's) Biosimilar pathway. Such ongoing approvals by the respective authorities are expected to drive the growth of insulin Biosimilar market. However, Insulin patent protection rights and strong retaliation from the branded manufactures has restricted the growth of insulin biosimilar development.
Request Free Report Sample@ http://www.futuremarketinsights.com/reports/sample/rep-gb-1883
Insulin Biosimilars Market: Segmentation
The global market for insulin Biosimilar is segmented on the basis of disease indication and geography.
On the basis of disease indication, the global insulin Biosimilar market is segmented as,
Type I Diabetes
Type II Diabetes
Insulin Biosimilars Market: Overview
Development of clinically proven insulin Biosimilar will pave the road for new entrants. The upcoming patent expiration of blockbuster insulin drugs will open a new avenue and unprecedented opportunities for biosimilar development and manufacturing companies. The cost of insulin biosimilar is currently high owing to stringent adherence requirements in terms of complex regulations and manufacturing and operations. Prices of biosimilar drugs are expected decreased by an average of 20% as compared to branded drugs.
Insulin Biosimilars Market: Region-wise Outlook
Geographically, the global insulin biosimilar market is segmented into regions namely, North America, Latin America, Western Europe, Eastern Europe, Asia-Pacific Excluding Japan, Japan, Middle East and Africa. Among all the regions, North America will continue to lead the global market for insulin biosimilars market due to high demand for insulin. Europe insulin market is expected to account for second largest share in global market primarily due to early approval for biosimilar which is expected to fuel the market growth. Asian companies are competing on the basis of price and has patient pool suffering diabetes. Hence, the Asia Pacific insulin biosimilar market is expected to witness fastest growth in overall market over the forecast period.
Visit For TOC@ http://www.futuremarketinsights.com/toc/rep-gb-1883
Insulin Biosimilars Market: Key Players
Key players operating in the global insulin biosimilar market are Eli Lilly & Co., Boehringer Ingelheim, Merck & Co., Pfizer Inc., Biocon, Mylan N.V. and others
About Us – Future Market Insights is the premier provider of market intelligence and consulting services, serving clients in over 150 countries. FMI is headquartered in London, the global financial capital, and has delivery centers in the U.S. and India.
Contact Us:
Future Market Insights
616 Corporate Way,
Suite 2-9018,
Valley Cottage,
New York 10989,
United States
Tel: +1-347-918-3531
Fax: +1-845-579-5705
Email: sales@futuremarketinsights.com
Website: www.futuremarketinsights.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Insulin Biosimilars Market Value Share, Supply Demand, share and Value Chain 2016-2026 here
News-ID: 401086 • Views: …
More Releases from Future Market Insights
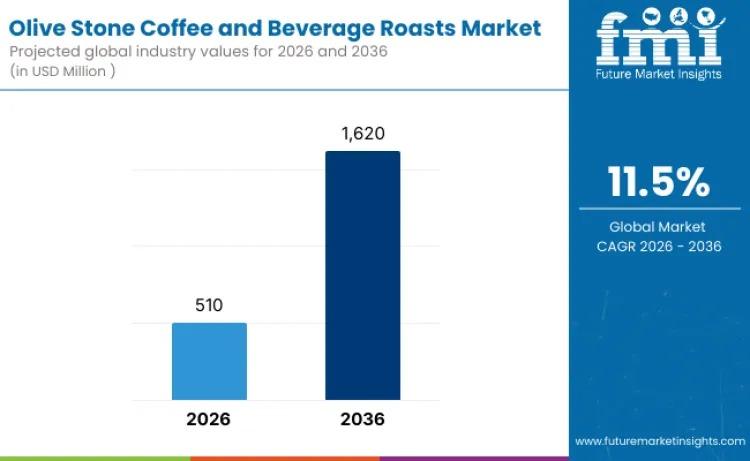
Global Olive Stone Coffee and Beverage Roasts Market to Reach USD 1,620 Million …
The global olive stone coffee and beverage roasts market is entering a high-growth decade, fueled by sustainability innovation and evolving specialty coffee culture. Valued at USD 510 million in 2026, the market is projected to reach USD 1,620 million by 2036, expanding at a compelling CAGR of 11.5%.
As consumers increasingly seek beverages that combine sustainability, functionality, and distinctive taste, olive stone-based roasting solutions are transitioning from niche experimentation to structured…
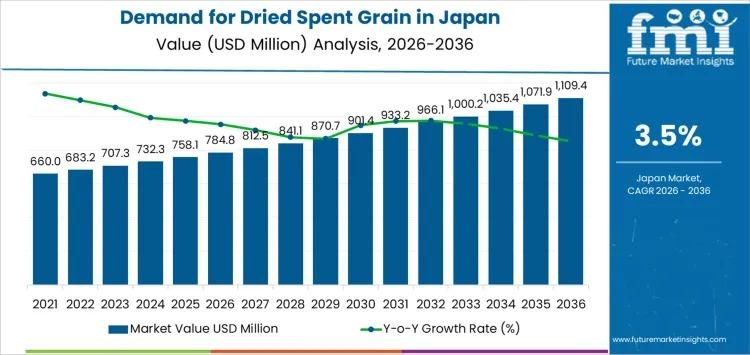
Japan Dried Spent Grain Market to Surpass USD 1.1 Billion by 2036 as Feed Optimi …
Japan's dried spent grain market is entering a decade of steady, value-driven expansion, supported by structured feed demand, brewery byproduct utilization, and rising integration of fiber-rich ingredients into food manufacturing. Industry estimates place the market at USD 784.8 million in 2026, with projections indicating growth to USD 1,109.4 million by 2036, reflecting a CAGR of 3.5%.
Between 2020 and 2026, demand increased from USD 637.5 million to USD 784.8 million, shaped…
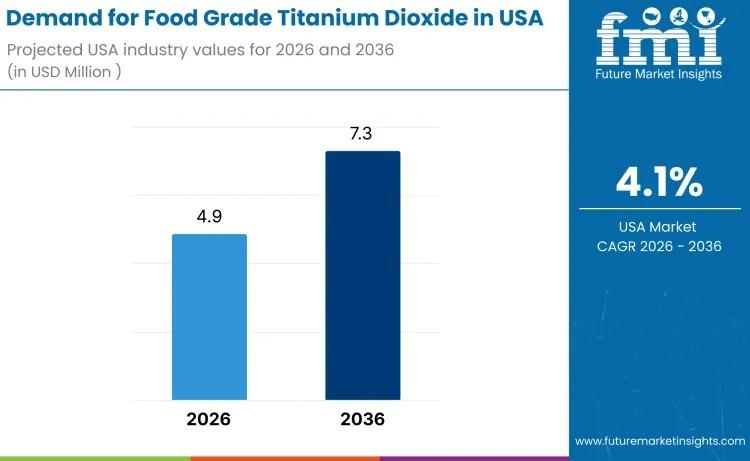
USA Food Grade Titanium Dioxide Market to Reach USD 7.3 Million by 2036 Amid Ste …
The demand for food grade titanium dioxide in the USA is valued at USD 4.9 million in 2026 and is projected to reach USD 7.3 million by 2036, expanding at a CAGR of 4.1%. Growth remains moderate yet stable, supported by continued use of titanium dioxide as a whitening and opacifying agent across confectionery coatings, bakery decorations, sauces, dairy analogues, and processed food matrices.
Despite heightened regulatory scrutiny and evolving clean-label…
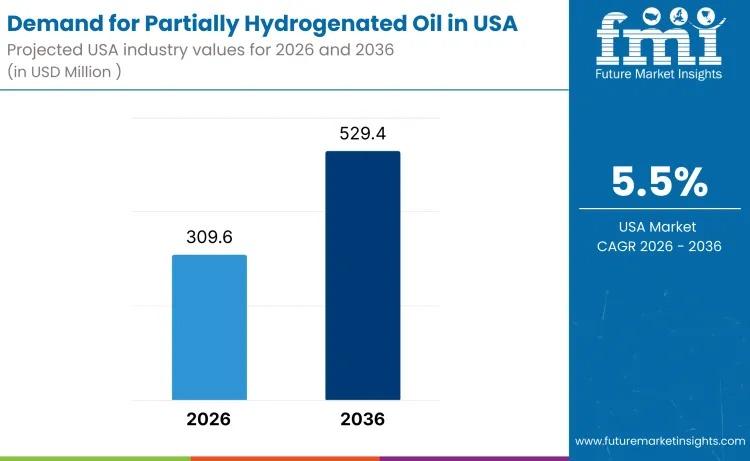
USA Partially Hydrogenated Oil Market to Reach USD 529.4 Million by 2036 Amid Me …
The demand for partially hydrogenated oil in the USA is projected to rise from USD 309.6 million in 2026 to USD 529.4 million by 2036, expanding at a steady CAGR of 5.5%. While edible applications remain tightly regulated, demand persists across specialty industrial and permitted food-related segments where oxidative stability, viscosity control, and texture performance remain critical.
Despite regulatory constraints on trans fats in conventional food manufacturing, PHOs continue to serve…
More Releases for Biosimilar
Interchangeable Biosimilar Humira Market Share Driven by Biologic Therapy Adopti …
Interchangeable Biosimilar Humira Market
The global market for Interchangeable Biosimilar Humira was valued at US$ million in the year 2024 and is projected to reach a revised size of US$ million by 2031, growing at a CAGR of %during the forecast period
View sample report
https://reports.valuates.com/request/sample/QYRE-Auto-33I15005/Global_Interchangeable_Biosimilar_Humira_Market_Research_Report_2023
The Interchangeable Biosimilar Humira Market is experiencing significant market growth as healthcare providers and patients increasingly adopt biosimilar therapies for autoimmune and inflammatory conditions. Market trends indicate rising…
Key Trend Reshaping the Biosimilar Monoclonal Antibodies Market in 2025: Advance …
What Are the Projections for the Size and Growth Rate of the Biosimilar Monoclonal Antibodies Market?
In recent times, the biosimilar monoclonal antibodies sector has experienced a swift expansion. The market size, which stands at $8.04 billion in 2024, is projected to climb to $9.25 billion in 2025, marking a compound annual growth rate (CAGR) of 15.1%. Factors such as expired patents, an increased understanding of biosimilars, governmental strategies, heightened financial…
Key Trend Reshaping the Biosimilar Monoclonal Antibodies Market in 2025: Advance …
What Are the Projections for the Size and Growth Rate of the Biosimilar Monoclonal Antibodies Market?
In recent times, the biosimilar monoclonal antibodies sector has experienced a swift expansion. The market size, which stands at $8.04 billion in 2024, is projected to climb to $9.25 billion in 2025, marking a compound annual growth rate (CAGR) of 15.1%. Factors such as expired patents, an increased understanding of biosimilars, governmental strategies, heightened financial…
Biosimilar Market Treating More for Less: The Booming Infliximab Biosimilar Mark …
Infliximab Biosimilar Market worth $ XX Million by 2030 - Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Infliximab Biosimilar Market- by Application (Crohn's Disease, Psoriatic Arthritis, Rheumatoid Arthritis, Ulcerative Colitis, Ankylosing Spondylitis, Plaque Psoriasis and Others), End User (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy and Other Direct Distribution Channels), Trends, Industry Competition Analysis, Revenue and Forecast To 2030."
Get…
Biosimilar Monoclonal Antibodies Market
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the " "Global Biosimilar Monoclonal Antibodies Market by Product (infliximab, trastuzumab, rituximab, adalimumab, bevacizumab, cetuximab, ranibizumab, denosumab, eculizumab, and other pipeline products), Indication (oncology, inflammatory & autoimmune disorders, chronic diseases, blood disorders, and other indications), Clinical Trial/Pipeline Analysis, Future Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
The Biosimilar Monoclonal Antibodies Market Size is valued at 5.02…
Infliximab Biosimilar Insight, 2022 | DelveInsight
DelveInsight's, "Infliximab Biosimilar Insight, 2022" report provides comprehensive insights about 35+ companies and 45+ marketed and pipeline drugs in Infliximab Biosimilars landscape. It covers the marketed and pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Interested to know more about the functioning of…
