Press release
Acquired Orphan Blood Disease Market Set to Expand Significantly with a 10.4% CAGR Through 2032, Driven by Advancements in Rare Disease Therapeutics and Global Awareness
Market Overview and Growth DynamicsThe global Acquired Orphan Blood Disease Market is poised for significant expansion, with an anticipated CAGR of 10.4% from 2022 to 2032. The market is expected to grow from a valuation of US$ 8 billion in 2022 to US$ 21.5 billion by 2032, driven by increased awareness of rare diseases, technological advancements in diagnostics, and a growing demand for specialized treatment options. The surge in chronic diseases across the globe, combined with rising healthcare expenditures, has further intensified the focus on orphan blood diseases, leading to more research and market entry.
Get a Sample PDF Brochure of the Report (Use Corporate Email ID for a Quick Response): https://www.persistencemarketresearch.com/samples/4464
Among all segments, Paroxysmal Nocturnal Hemoglobinuria (PNH) emerges as a leading indication, largely due to its severity and growing diagnostic awareness. North America holds the lion's share of the global market, contributing approximately 37.5% in 2021. This dominance is attributed to a high incidence of rare blood disorders, robust healthcare infrastructure, and proactive governmental initiatives supporting orphan drug development. The U.S. continues to be a key revenue generator, backed by strong R&D pipelines and increased insurance coverage for rare conditions.
✦ Key Highlights from the Report
✦ Market forecast anticipates growth from US$ 8 Bn in 2022 to US$ 21.5 Bn by 2032.
✦ North America held a 37.5% revenue share of the global market in 2021.
✦ Paroxysmal Nocturnal Hemoglobinuria (PNH) is the leading disease segment.
✦ Increasing chronic disease prevalence drives demand for orphan blood treatments.
✦ Technological advancements in diagnostics boost early identification and treatment.
✦ Emerging markets like India offer promising opportunities due to rising incomes.
Market Segmentation
The acquired orphan blood disease market is segmented based on disease type, treatment type, and end user. Disease-wise segmentation includes PNH, Myelodysplastic Syndrome (MDS), Idiopathic Thrombocytopenic Purpura (ITP), Myelofibrosis (MF), and Polycythemia Vera (PV). PNH remains the most dominant due to the severity of anemia and associated complications. MDS and ITP follow due to their rising detection rates.
From the treatment perspective, the market is categorized into blood transfusion, bone marrow transplant, iron therapy, and pharmacological treatment. Among these, pharmacological therapy is the leading segment due to increased drug development, ease of administration, and availability of targeted therapies. Bone marrow transplantation, while effective, is limited by donor availability and cost.
End-user segmentation includes hospitals, specialty clinics, and academic research institutes. Hospitals dominate due to their capability to handle complex procedures like transfusions and bone marrow transplants. Specialty clinics are expected to grow rapidly with the advancement of outpatient care facilities for rare diseases.
Regional Insights
North America remains at the forefront, driven by high awareness, government support for orphan diseases, advanced healthcare systems, and leading pharmaceutical companies investing in R&D. Additionally, rising access to healthcare insurance in the U.S. has boosted diagnosis and treatment rates.
Europe follows closely with substantial investment in rare disease research and collaborative efforts among EU nations to create orphan drug pipelines. Countries like Germany and the UK are significantly investing in clinical trials and expanding patient registries.
Asia Pacific is rapidly emerging as a high-growth region due to increasing public and private investments in healthcare infrastructure, growing awareness of blood-related disorders, and an expanding middle-class population willing to invest in advanced treatment. Particularly, India and China are projected to witness strong growth, backed by supportive government health initiatives and clinical research investments.
Latin America and the Middle East & Africa are also gradually witnessing progress, with growing international partnerships and access to previously unavailable orphan drugs.
Market Drivers
The primary market driver is the rising incidence of chronic and rare blood disorders, prompting the need for targeted therapies. Improved diagnostic technologies allow for earlier and more accurate detection of diseases like PNH, MF, and MDS. Additionally, increased per capita income in developing countries is expanding the affordability of advanced treatment options, contributing to broader market penetration. Governmental policies supporting orphan drug development-including tax incentives and exclusivity rights-also play a pivotal role.
Market Restraints
One of the key challenges in this market is the lengthy and complex regulatory process required for drug approvals. Orphan drugs, although necessary, undergo rigorous testing and trials, often prolonging their time-to-market. Stringent healthcare regulations across various regions further delay product availability. Another barrier is limited investment in the development of treatments for rare diseases due to the smaller patient pool, which may not promise high commercial returns. Additionally, market consolidation reduces the entry of innovative startups and new therapies.
Market Opportunities
The market offers promising opportunities with the emergence of novel therapies and gene editing technologies, particularly those targeting bone marrow function and genetic mutations responsible for orphan blood diseases. Increased global collaboration in clinical trials and patient data sharing also paves the way for faster drug discovery. Pharmaceutical companies entering emerging markets like India, China, and Brazil are expected to benefit from large, underdiagnosed patient populations and rising healthcare investments. Public-private partnerships are likely to further boost development.
# Frequently Asked Questions (FAQs)
How Big is the Acquired Orphan Blood Disease Market?
Who are the Key Players in the Global Market for Acquired Orphan Blood Disease?
What is the Projected Growth Rate of the Market?
What is the Market Forecast for Acquired Orphan Blood Disease Through 2032?
Which Region is Estimated to Dominate the Industry through the Forecast Period?
Company Insights
• Alexion Pharmaceuticals, Inc.
• Amgen, Inc.
• Celgene Corporation
• Eli Lilly and Company
• Sanofi S.A.• GlaxoSmithKline plc
• Cyclacel Pharmaceuticals, Inc.
• Onconova Therapeutics, Inc.
• Incyte Corporation
• CTI BioPharma Corp.
Recent Developments:
In 2023, Alexion Pharmaceuticals launched a new therapy targeting complement pathways for patients with PNH, accelerating market penetration.
Incyte Corporation announced Phase III trial results for a novel JAK1/2 inhibitor, showing promise in treating Myelofibrosis with reduced side effects.
Conclusion
The Acquired Orphan Blood Disease Market stands at a pivotal point of transformation, driven by advances in biotechnology, an expanding global patient base, and supportive policy frameworks. While challenges like regulatory bottlenecks and limited investments persist, the trajectory remains overwhelmingly positive. Stakeholders who actively invest in research, public awareness, and strategic expansion into emerging markets are expected to thrive. With continued innovation and global collaboration, the market is set to revolutionize treatment for rare blood disorders, offering renewed hope for millions worldwide.
Persistence Market Research
G04 Golden Mile House, Clayponds Lane
Brentford, London, TW8 0GU UK
USA Phone: +1 646-878-6329
UK Phone: +44 203-837-5656
Email: sales@persistencemarketresearch.com
Web:
https://www.persistencemarketresearch.com
About Persistence Market Research:
At Persistence Market Research, we specialize in creating research studies that serve as strategic tools for driving business growth. Established as a proprietary firm in 2012, we have evolved into a registered company in England and Wales in 2023 under the name Persistence Research & Consultancy Services Ltd. With a solid foundation, we have completed over 3600 custom and syndicate market research projects, and delivered more than 2700 projects for other leading market research companies' clients.
Our approach combines traditional market research methods with modern tools to offer comprehensive research solutions. With a decade of experience, we pride ourselves on deriving actionable insights from data to help businesses stay ahead of the competition. Our client base spans multinational corporations, leading consulting firms, investment funds, and government departments. A significant portion of our sales comes from repeat clients, a testament to the value and trust we've built over the years.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Acquired Orphan Blood Disease Market Set to Expand Significantly with a 10.4% CAGR Through 2032, Driven by Advancements in Rare Disease Therapeutics and Global Awareness here
News-ID: 4000294 • Views: …
More Releases from Persistence Market Research
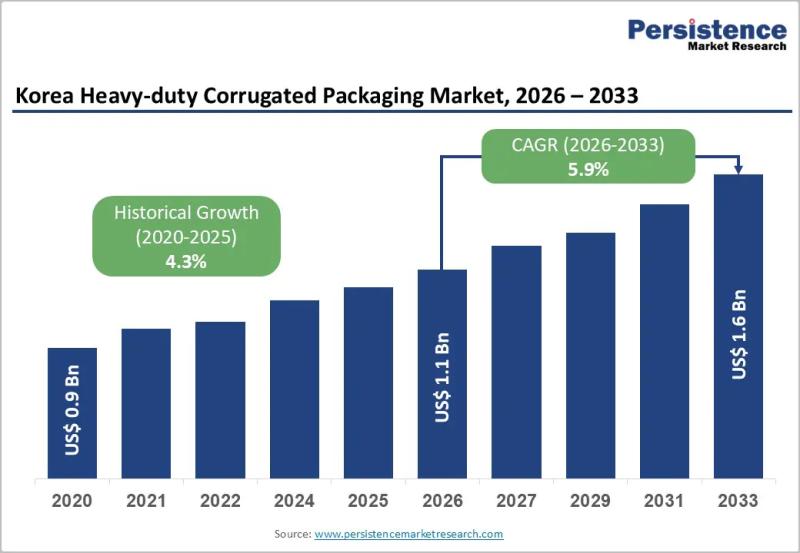
Korea Heavy-duty Corrugated Packaging Market to Reach US$1.6 Billion by 2033 - P …
The Korea heavy-duty corrugated packaging market plays a critical role in supporting industrial logistics, bulk transportation, and export-driven manufacturing. Heavy-duty corrugated packaging is widely used for shipping machinery, automotive components, electronics, chemicals, and large industrial goods that require superior strength and structural integrity. Unlike conventional corrugated boxes, heavy-duty variants are engineered with multi-wall boards, reinforced liners, and customized structural designs to withstand high load capacity, stacking pressure, and long-distance transportation.…

Textile Flooring Market Set for Steady Growth as Demand for Sustainable and Styl …
The global textile flooring market is entering a phase of stable expansion, supported by rising construction activity, increasing consumer focus on interior aesthetics, and growing demand for eco-friendly flooring solutions. According to industry estimates, the global textile flooring market size is likely to be valued at US$11.1 billion in 2026 and is projected to reach US$16.5 billion by 2033, expanding at a CAGR of 5.8% between 2026 and 2033. This…

Power System Simulator Market Size to Reach US$ 2.6 Billion by 2033 - Persistenc …
The power system simulator market is gaining strategic importance as global energy systems transition toward digitalization, decentralization, and decarbonization. Power system simulators are advanced software and hardware platforms used by utilities, grid operators, engineering firms, and research institutions to model, analyze, and optimize electrical power networks. These simulators enable real time grid analysis, contingency planning, load flow studies, fault analysis, stability assessment, and operator training. As electricity networks become more…

Yoga and Meditation Products Market Set for Robust Growth, Projected to Reach US …
The global wellness industry is undergoing a major transformation as consumers increasingly prioritize mental health, mindfulness, and preventive self-care. Within this evolving landscape, the yoga and meditation products market has emerged as a fast-growing segment, encompassing everything from yoga mats and apparel to meditation cushions, smart devices, and digital-enabled accessories. According to industry estimates, the global yoga meditation products market is projected to be valued at US$ 8.3 billion in…
More Releases for Orphan
Acquired Orphan Blood Disease Market
Acquired Orphan Blood Disease Market to reach over USD 18.93 billion by the year 2031 - Exclusive Report by InsightAce Analytic
According to a new report by InsightAce Analytic, the "Acquired Orphan Blood Disease Market" in terms of revenue was estimated to be worth $8.65 billion in 2023 and is poised to reach $18.93 billion by 2031, growing at a CAGR of 10.47% from 2024 to 2031.
Get Free Access to…
Orphan Drugs Market Size to Hit $3199.3 Billion by 2028 | Orphan Drugs Industry …
Market Overview:
According to our experience research team, Orphan Drugs Market was valued at USD 112.36 Billion in 2021, and the global Orphan Drugs industry is projected to reach a value of USD 3199.3 Billion by 2028, at a CAGR of 7.4% during the forecast period 2022-2028
Vantage Market Research is a collection of market research studies on several industries, such as Chemicals, semiconductors & Electronics, Food & Beverages Technology, Energy &…
Orphan Drugs for Cancer Pipeline Analysis
A huge market opportunity is offered by small patient population which suffers from rare or orphan diseases. Among the category of new orphan drugs, Oncology account for the largest disease group in recent years. It has been observed that majority of the orphan drugs in the clinical stages are for rare cancer disease drugs, and are in the late stages of the pipeline. Some of the drugs are being developed…
US Orphan Drug Pipeline Analysis
In recent years, the pharmaceutical industry has been experiencing a paradigm shift. While a large pool of patients was considered as a major source of revenue for pharma companies in the past, the focus is now gradually shifting to small sections of patients suffering from rare disease. In US, this pool of patients is gradually growing and orphan drugs are becoming an extremely attractive business proposition for the pharmaceuticals industry.…
Europe Orphan Drugs Pipeline Analysis
“Europe Orphan Drugs Pipeline Analysis” by PNS Pharma gives comprehensive insight on the various drug profiles under Orphan Drugs status in Europe. Research report covers all the ongoing drug development in various phases. Each drug profiles include detailed information like: Originator, Owner, Collaborator, Technology Provider, Licensee, Development Phase, Development Indications, Mechanism of Action, Chemical Formula, Country of Development and detailed analysis on the development process. The information for particular drug…
Global Orphan Drug Pipeline Analysis
In recent years, the pharmaceutical industry has been experiencing a paradigm shift. While a large pool of patients was considered as a major source of revenue for pharma companies in the past, the focus is now gradually shifting to small sections of patients suffering from rare disease. In US & Europe, this pool of patients is gradually growing and orphan drugs are becoming an extremely attractive business proposition for the…
