Press release
Clinical Trials Manufacturing and Supply Outsourcing Market Report on the Untapped Growth Opportunities in the Industry
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trials Manufacturing and Supply Outsourcing Market- (By Service Type (Manufacturing, Logistics & Distribution, Storage & Retention, Others), By Phase Type (Phase-I, Phase-II, Phase-III, Phase-IV), By End-User (Pharmaceutical Companies, Biotechnology Companies, Others)), Trends, Industry Competition Analysis, Revenue and Forecast To 2031."According to the latest research by InsightAce Analytic, the Global Clinical Trials Manufacturing and Supply Outsourcing Market is expected to show a CAGR of 7.28% during a forecast period of 2023-2031.
Request for free Sample Pages:
https://www.insightaceanalytic.com/request-sample/2158
Clinical trials manufacturing and supply outsourcing involves a strategic collaboration between pharmaceutical and biotechnology firms and specialized contract manufacturing organizations (CMOs) or contract development and manufacturing organizations (CDMOs). The market is primarily driven by the increasing number of clinical trials, regulatory harmonization, rising research and development investments from pharmaceutical and biotech companies, and advancements in supply chain technologies. Outsourcing these services is becoming increasingly common as the costs associated with clinical studies continue to rise. Moreover, the rapid growth of biotechnology has led to a surge in biopharmaceutical-related clinical trials, creating new opportunities in the global market. Biotech companies require specialized supplies tailored for biologics and cell therapies, further driving the demand for outsourcing services.
Additionally, the emergence of patient-centric clinical trials, which prioritize enhancing the patient experience and engagement, has led to the demand for innovative supply solutions. These include home-based trials, remote monitoring, and direct-to-patient delivery services. As personalized medicine and precision therapies gain traction, the need for tailored clinical trial supplies-aligned with individual patient profiles-has further fueled the demand for customized supply solutions.
List of Prominent Players in the Clinical Trials Manufacturing and Supply Outsourcing Market:
• Catalent
• Parexel
• Almac Group Limited
• Patheon
• Thermo Fischer Scientific
• Klifo
• Movianto
• Eurofins Scientific
• Clinigen
• Spaulding Clinical
• Singota
• Symeres
• Ardena
• Others
Market Dynamics:
Drivers-
Chronic diseases, driven by the ever-growing global population and rising infections, pose a significant burden on healthcare systems, and play a crucial role in drug development clinical trials. For a drug to be approved for human use, it must successfully navigate all standard clinical trial phases, ensuring its safety for treating these long-term illnesses. The increasing prevalence of chronic conditions such as HIV, cancer, and epilepsy has spurred growth in the clinical trial supply market. This sector is further bolstered by the growing focus on evaluating toxicity levels in the early stages of drug development, along with an increasing demand for outsourced drug discovery services.
Curious about this latest version of the report? Enquiry Before Buying:
https://www.insightaceanalytic.com/enquiry-before-buying/2158
Challenges:
A major challenge facing the global clinical trial supply market is the rising cost of clinical trials. The need for more extensive clinical data collection has significantly contributed to the escalating costs. This, combined with the high research and development expenses once a clinical trial is approved, represents a considerable obstacle to market growth. Additionally, there are other emerging trends and challenges, including regulatory hurdles and the complexity of global trials, that may impact the industry.
Regional Trends:
North America is projected to hold a significant share of the Clinical Trials Manufacturing and Supply Outsourcing Market, with strong growth expected in the coming years. The global expansion of clinical trials and substantial investments in research and development (R&D) are key factors driving the growth of this market in North America. The region benefits from a high concentration of Contract Research Organizations (CROs) and an increasing number of registered clinical trials. Major players like Pfizer, Abbott Labs, and Johnson & Johnson dominate the market in this region. Looking ahead, the Asia Pacific region is expected to experience substantial growth, fueled by the increasing number of healthcare corporations conducting clinical trials, favorable government regulations, and the availability of affordable treatments. The region is set to emerge as one of the fastest-growing markets for clinical trial supplies.
Recent Developments:
• In Sept. 2023, The recent partnership between Thermo Fisher Scientific, Inc. and the National Minority Quality Forum (NMQF) through the NMQF's Alliance for Representative Clinical Trials (ARC) aims to facilitate access to clinical research for patient populations that have been historically underserved.
• In May 2020, Teva-Takeda Pharmaceuticals, Nagoya Aichi, Japan, and Catalent have reached an agreement for the acquisition of a clinical packaging facility in Minakuchi, Shiga prefecture... A new clinical GMP manufacturing and distribution centre will be established through this acquisition in order to support clinical studies.
Get Specific Chapter/Information From The Report:
https://www.insightaceanalytic.com/customisation/2158
Segmentation of Clinical Trials Manufacturing and Supply Outsourcing Market-
By Service Type-
• Manufacturing
• Logistics & Distribution
• Storage & Retention
• Others
By Phase Type-
• Phase-I
• Phase-II
• Phase-III
• Phase-IV
By End-User-
• Pharmaceutical Companies
• Biotechnology Companies
• Others
By Region-
North America-
• The US
• Canada
• Mexico
Europe-
• Germany
• The UK
• France
• Italy
• Spain
• Rest of Europe
Asia-Pacific-
• China
• Japan
• India
• South Korea
• Southeast Asia
• Rest of Asia Pacific
Latin America-
• Brazil
• Argentina
• Rest of Latin America
Middle East & Africa-
• GCC Countries
• South Africa
• Rest of Middle East and Africa
Get more information:@
https://www.insightaceanalytic.com/report/clinical-trials-manufacturing-and-supply-outsourcing-market/2158
About Us:
InsightAce Analytic is a market research and consulting firm that enables clients to make strategic decisions. Our qualitative and quantitative market intelligence solutions inform the need for market and competitive intelligence to expand businesses. We help clients gain a competitive advantage by identifying untapped markets, exploring new and competing technologies, segmenting potential markets, and repositioning products. Our expertise is in providing syndicated and custom market intelligence reports with an in-depth analysis with key market insights in a timely and cost-effective manner.https://www.insightaceanalytic.com/images_data/148861653.JPG
Contact us:
info@insightaceanalytic.com
InsightAce Analytic Pvt. Ltd.
Visit: www.insightaceanalytic.com
Tel : +1 551 226 6109
Asia: +91 79 72967118
Follow Us on LinkedIn @ bit.ly/2tBXsgS
Follow Us On Facebook @ bit.ly/2H9jnDZ
Twitter: https://twitter.com/Insightace
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Clinical Trials Manufacturing and Supply Outsourcing Market Report on the Untapped Growth Opportunities in the Industry here
News-ID: 3992482 • Views: …
More Releases from Insightace Analytic Pvt Ltd.
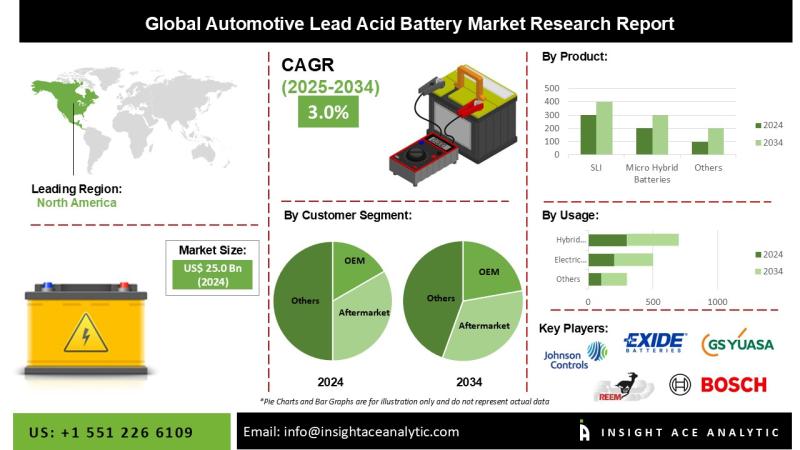
Automotive Lead Acid Battery Market Strategic Growth Drivers and Outlook 2026 to …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Automotive Lead Acid Battery Market Size, Share & Trends Analysis Report By Product (SLI and Micro-Hybrid Batteries), Type (Flooded, Enhanced Flooded, and VRLA), Customer Segment (OEM and Aftermarket), End User (Passenger Car, Light Commercial Vehicles, Heavy Commercial Vehicles, Two-Wheeler, and Three-Wheeler), and Application (Hybrid Vehicles, Electric Vehicles, Light Motor Vehicles, and Heavy Motor Vehicles)- Market…
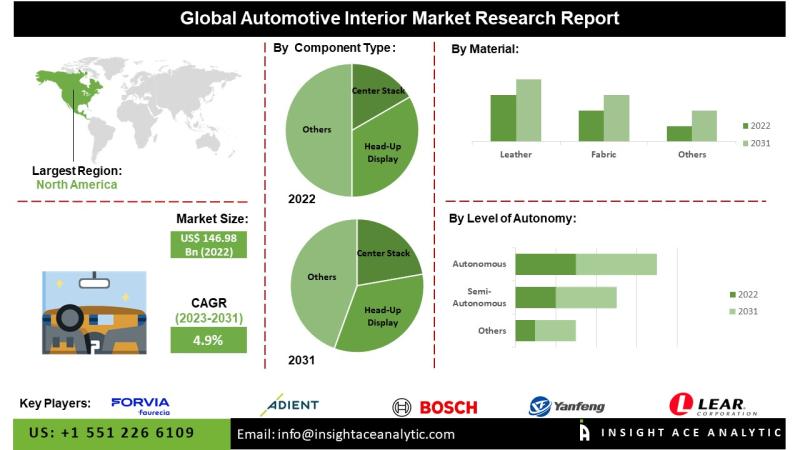
Automotive Interior Market Investment Opportunities and Forecast 2026 to 2035
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Automotive Interior Market- (By Component Type (Center Stack, Head-up Display, Instrument Cluster, Rear Sear Entertainment, Dome Module, Headliner, Seat, Interior Lighting Door Panel, Center Console, Adhesives & Tapes, Upholstery, Others), By Material (Leather, Fabric, Vinyl, Wood, Glass Fiber Composite, Carbon Fiber Composite, Metal), By Level of Autonomy (Semi-Autonomous, Autonomous, Non-Autonomous),By Electric Vehicle (Battery Electric Vehicle…
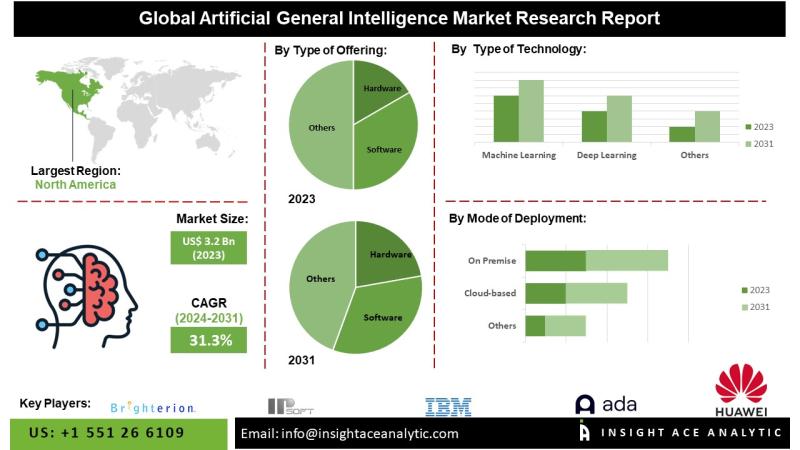
Artificial General Intelligence Market Future Landscape and Industry Evolution 2 …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Artificial General Intelligence (AGI) Market - (By Type of Offering (Hardware, Software and Service), Type of Technology (Machine Learning, Deep Learning, Natural Language Processing and Robotics), Mode of Deployment (Cloud-based, On Premise and Web-based), Type of AI (Weak AI, Strong AI and Superintelligence), Type of Processing (Image, Text and Voice Processing), Company Size (SMEs and…
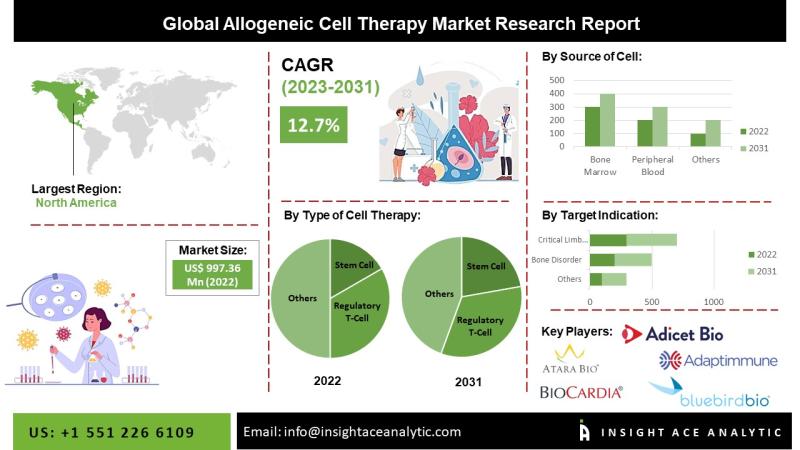
Allogenic Cell Therapies Market Revenue Trends and Growth Potential 2026 to 2035
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Allogenic Cell Therapies Market- by Cell Type(Cardiosphere-Derived Cells (CDCs), Fibroblasts, T-cells, Mesenchymal Stem Cells (MSCs), Hematopoietic Stem Cells (HSCs) and Others),Tissue Source(Skin, Blood, PBC, BM and Others), Indication (Acute graft-versus-host disease (GVHD), Chronic Ulcers and Diabetic Foot Ulcers, Osteoarthritis, Crohn's Disease, Cardiovascular Disease, Solid Tumors/Cancers and Others (Alzheimer's Disease, etc.)), Trends, Industry Competition Analysis, Revenue…
More Releases for Clinical
Miami Clinical Research Sets the Standard for Clinical Trials
Miami Clinical Research, a frontrunner in the world of clinical trials and medical research, has emerged as the prime choice for global corporate pharmaceutical giants. With a deep understanding of the complexities of medical studies, the organization champions the crucial role of research in the evolution of transformative therapeutic interventions.
Miami, FL - Renowned as a first-rate center for professional medical exploration, Miami Clinical Research [https://miamiclinicalresearch.com] boasts state-of-the-art facilities, advanced technologies,…
E-Clinical Solutions Market: Revolutionizing Healthcare and Clinical Trials
Introduction
The e-Clinical solutions market has become a pivotal component of the healthcare and pharmaceutical industries. E-Clinical solutions refer to a set of software, tools, and platforms designed to streamline clinical trials and healthcare management. These solutions include electronic data capture (EDC), clinical trial management systems (CTMS), laboratory information management systems (LIMS), and other integrated tools that improve the efficiency, accuracy, and speed of clinical trials and healthcare services. The primary…
E-Clinical Solutions Market: Revolutionizing Clinical Trials
The e-clinical solutions market has experienced significant growth in recent years, driven by the increasing complexity of clinical trials and the need for efficient, accurate, and compliant data management. E-clinical solutions provide a comprehensive suite of tools and technologies to streamline clinical trial processes, accelerate drug development, and improve patient outcomes.
Market Size and Growth
The global e-clinical solutions market is estimated to be worth billions of dollars, with a significant portion…
Clinical Trials Management System Market Optimizing Clinical Trials: The Crucial …
Clinical Trials Management System Market to reach over USD 5.06 billion by the year 2031- Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trials Management System Market Size, Share & Trends Analysis Report By Solution Type (Enterprise and Site based), By Delivery Mode (Web & Cloud-based, On-premise), By Component (Software, Services), By End-user (Pharmaceutical and Biotechnology Firms, Medical…
Clinical Research and Clinical Trials Summit
Clinical Research 2019 has been designed in an interdisciplinary manner with a multitude of tracks to choose from every segment and provides you with a unique opportunity to meet up with peers from both industry and academia and establish a scientific network between them. We cordially invite all concerned people to come join us at our event and make it successful by your participation.
This is the premier interdisciplinary forum for…
E-Clinical Trial Solutions Market To Accelerating Clinical Development Technolog …
The study of the "Global e-Clinical Trial Solutions Market" provides the market size information and market trends along with the factors and parameters impacting it in both short and long term. The study ensures a 360° view, bringing out the complete key insights of the industry.
The Global e-Clinical Trial Solutions Market Research Report Forecast 2017-2021 is a valuable source of insightful data for business strategists. It provides the e-Clinical…
