Press release
GMP Biologics Market expected to Witness Huge Revenue Growth to 2031
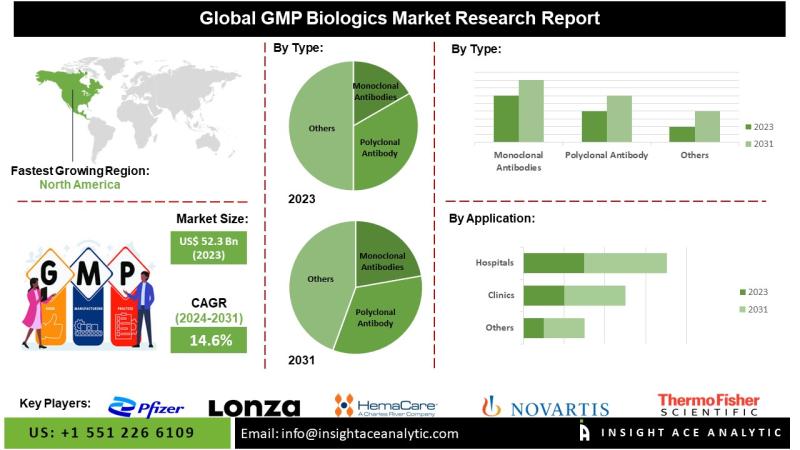
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "GMP Biologics Market"-, By Type (Monoclonal Antibodies, Polyclonal Antibody), By Application (Hospitals, Clinics, Others), Industry Trends, and Global Forecasts, 2024-2031 And Segment Revenue and Forecast To 2031."The GMP Biologics Market is estimated to reach over USD 153.9 Bn by 2031, exhibiting a CAGR of 14.6% during the forecast period.
Get Free Access to Demo Report, Excel Pivot and ToC: https://www.insightaceanalytic.com/request-sample/2715
Good Manufacturing Practices (GMP) represent a framework of rigorous guidelines and operational protocols applied during the production of pharmaceuticals, biologics, and medical products to ensure product safety, quality, and consistency. Biologic therapies-originating from living organisms-play a crucial role in modulating or enhancing physiological functions. These include treatments such as hormone replacements for endocrine conditions and vaccines that stimulate the immune system.
GMPs are instrumental in mitigating risks related to contamination, manufacturing errors, and instability of biologic products. Due to their biological origins, these therapies are more intricate than conventional small-molecule drugs, with their development influenced by various biological and environmental factors, including genetic variability, exposure to pathogens, and naturally occurring biochemical impurities. These complexities make the production of biologics particularly challenging, necessitating strict adherence to GMP protocols across all stages of development and manufacturing.
List of Prominent Players in the GMP Biologics Market:
• Amgen Inc.
• F. Hoffmann-La Roche Ltd
• AbbVie Inc.
• AstraZeneca plc
• Merck KGaA
• Creative Diagnostics
• Fisher Bioservices (Thermo Fisher Scientific)
• Polpharma Biologics
• Intertek
• HemaCare
• AGC
• AstraZeneca plc
• Merck KGaA
• Sanofi
• GlaxoSmithKline plc
• Johnson & Johnson
• Pfizer Inc.
• Novartis AG
• Eli Lilly and Company
• Samsung Biologics Co. Ltd.
• WuXi AppTec
• Lonza Group Ltd.
• Rentschler Biopharma SE
• Boehringer Ingelheim International GmbH
• Celltrion Inc
• Catalent Inc.
Market Dynamics
Drivers:
The surging global demand for biopharmaceuticals-including therapeutic proteins, monoclonal antibodies, and vaccines-is a key factor propelling the growth of the GMP-compliant biologics manufacturing sector. These biologics provide precise and highly effective treatment options across a spectrum of medical conditions, prompting greater emphasis on stringent production standards to ensure quality, safety, and efficacy. The increasing incidence of chronic illnesses such as cancer, autoimmune diseases, and infectious disorders has intensified the need for advanced biologic therapies, further amplifying the requirement for GMP-aligned production practices.
Enquiry Before Buying:
https://www.insightaceanalytic.com/customisation/2715
Challenges:
One of the most pressing challenges in biologics production is maintaining aseptic conditions across all stages of the manufacturing lifecycle. The complex and sensitive nature of biologic products makes them particularly susceptible to contamination, thereby requiring rigorous adherence to GMP protocols. Continuous environmental monitoring and stringent quality assurance measures are critical to safeguarding product integrity and protecting patient health.
Regional Trends: North America is projected to maintain a leading position in the GMP biologics market over the forecast period. This dominance is attributed to the region's concentration of major biopharmaceutical players, cutting-edge manufacturing infrastructure, and supportive regulatory frameworks-particularly the guidance provided by the U.S. Food and Drug Administration (FDA). The U.S. remains at the forefront of innovation in biologic therapies, including next-generation treatments such as gene therapies and monoclonal antibodies.
Meanwhile, the Asia-Pacific region is experiencing a notable acceleration in market growth. This expansion is driven by rising healthcare demands, substantial investment in biotech R&D, and growing regional expertise in biologics manufacturing processes.
Recent Developments:
• In Feb 2024, Abbie established a strategic relationship with Tentarix Biotherapeutics to explore and create novel, multi-specific, conditionally active biologic candidates in immunology and oncology.
• In June 2023, Wuxi Biologics, which provides comprehensive solutions for biologics R&D and manufacturing, has declared that it can now produce more therapeutic components and pharmaceuticals at its plant in Wuppertal, Germany.
• In March 2023, Samsung Biologic, Incheon, South Korea's Samsung Biologics is undergoing planned expansion in response to increasing market demand. When the facility is completed, it is expected to have a manufacturing capacity of 180,000 L. The firm plans to invest KRW 1.9 trillion to boost the site's capacity to 784,000 L.
Unlock Your GTM Strategy:
https://www.insightaceanalytic.com/customisation/2715
Segmentation of GMP Biologics Market.
Global GMP Biologics Market- By Type
• Monoclonal Antibodies
• Polyclonal Antibody
Global GMP Biologics Market - By Application
• Hospitals
• Clinics
• Others
Global GMP Biologics Market - By Region
North America-
• The US
• Canada
• Mexico
Europe-
• Germany
• The UK
• France
• Italy
• Spain
• Rest of Europe
Asia-Pacific-
• China
• Japan
• India
• South Korea
• Southeast Asia
• Rest of Asia Pacific
Latin America-
• Brazil
• Argentina
• Rest of Latin America
Middle East & Africa-
• GCC Countries
• South Africa
• Rest of the Middle East and Africa
Get more information:
https://www.insightaceanalytic.com/report/gmp-biologics-market/2715
About Us:
InsightAce Analytic is a market research and consulting firm that enables clients to make strategic decisions. Our qualitative and quantitative market intelligence solutions inform the need for market and competitive intelligence to expand businesses. We help clients gain a competitive advantage by identifying untapped markets, exploring new and competing technologies, segmenting potential markets, and repositioning products. Our expertise is in providing syndicated and custom market intelligence reports with an in-depth analysis with key market insights in a timely and cost-effective manner.https://www.insightaceanalytic.com/images_data/148861653.JPG
Contact us:
info@insightaceanalytic.com
InsightAce Analytic Pvt. Ltd.
Visit: www.insightaceanalytic.com
Tel : +1 551 226 6109
Asia: +91 79 72967118
Follow Us on LinkedIn @ bit.ly/2tBXsgS
Follow Us On Facebook @ bit.ly/2H9jnDZ
Twitter: https://twitter.com/Insightace
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release GMP Biologics Market expected to Witness Huge Revenue Growth to 2031 here
News-ID: 3983840 • Views: …
More Releases from Insightace Analytic Pvt Ltd.
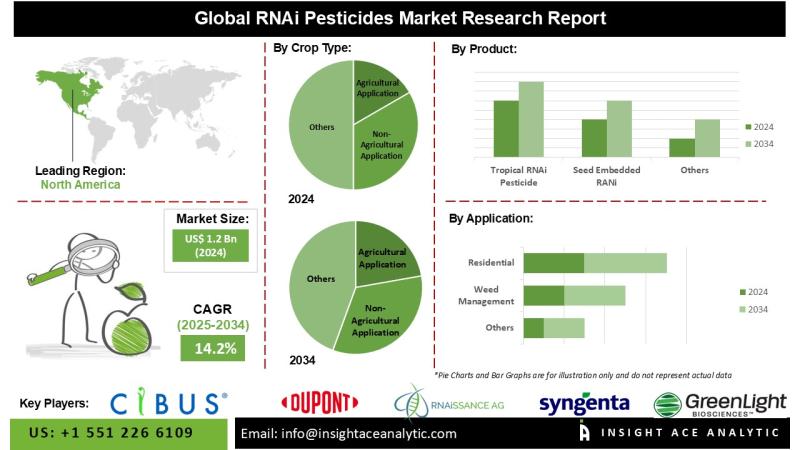
RNAi Pesticides Market Report on the Untapped Growth Opportunities in the Indust …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "RNAi Pesticides Market"-, By Application (Insect Pest Control, Weed Management, Disease Management, Resistance Management), By Crop Type (Agricultural Application, Non-Agricultural Application), By Product (Tropical RNAi Pesticide, Seed Embedded RANi, Transgenic RNAi, Others), By Formulation (Liquid Formulation, Granular Formulation, Powder Formulation, Others), Industry Trends, and Global Forecasts, 2025-2034 And Segment Revenue and Forecast To 2034."
The RNAi…
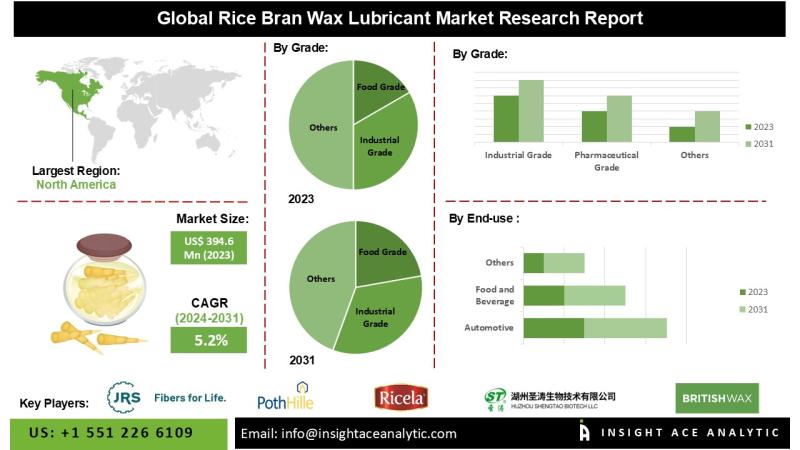
Rice Bran Wax Lubricant Market Know the Scope and Trends
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Rice Bran Wax Lubricant Market - (By Grade (Food Grade, Industrial Grade, Pharmaceutical Grade), End-use (Automotive, Food and Beverage, Cosmetics and Personal Care, Pharmaceuticals, Chemicals, Others)), Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
According to the latest research by InsightAce Analytic, the Global Rice Bran Wax Lubricant Market is valued at US$ 394.6 million…
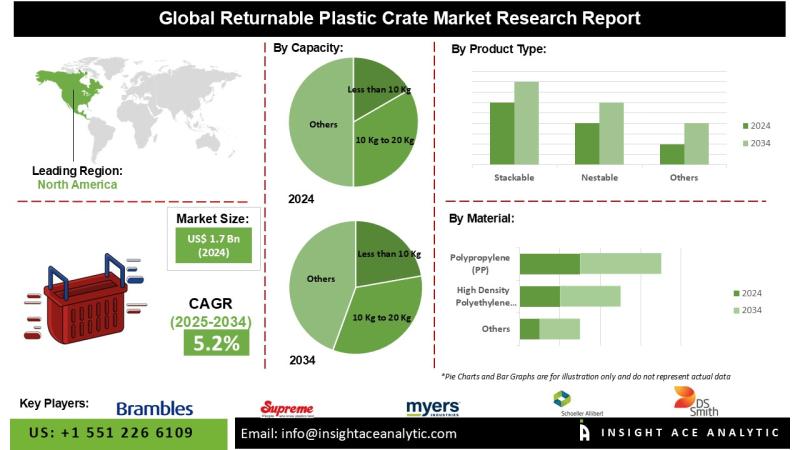
Returnable Plastic Crate Market Exclusive Report with Detailed Study Analysis
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Returnable Plastic Crate Market - (By Capacity (Less than 10 kg, 10 kg to 20 kg, 21 to 35 kg, 36 to 50 kg, Above 50 kg), By Product Type (Stackable, Nestable, Collapsible), By Material (High-Density Polyethylene (HDPE), Polypropylene (P.P.), Others), By Application (Agriculture, Grocery, Dairy, Bakery, Seafood & Meat, Others)), Trends, Industry Competition…
Respiratory Disease Genetic Testing Market Exclusive Report on the Latest Revenu …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Respiratory Disease Genetic Testing Market- (By Offerings (Products (Kits and Consumables), Services, Others), By Disease Type (COPD (Alpha-1-antitrypsin (AAT), Cystic Fibrosis (CF), Diffuse Lung Disease/Surfactant Dysfunction (RHD (Respiratory Distress Syndrome), PPHN (Persistent Pulmonary Hypertension of the Newborn)), Interstitial lung disease, Pulmonary Arterial Hypertension, Pulmonary Hypoplasia, Primary Ciliary Dyskinesia, Other Diseases (BPD)), By Offerings (PCR, NGS…
More Releases for GMP
Creative Peptides Released GMP Synthesis Service
Located in Shirley, New York, the world’s leading peptide supplier Creative Peptides announced the launch of its GMP synthesis (https://www.creative-peptides.com/services/custom-gmp-peptide-synthesis-services.html ) business on August 29, 2018. Now this company is focused on the development and GMP manufacturing of pharmaceutical grade peptides.
As the demand of pharmaceutical market continues to grow, more and more pharmas and research institutions choose the CMO and CRO models to expand their businesses, which is more…
Diapharm implements European GMP guidelines in China
Münster (DE), London (UK), Ningbo (CN), 20 December 2013 – Pharmaceutical service provider Diapharm (diapharm.com) is increasing its business activities in China: Diapharm has now implemented a “European” quality management system for Neptune Pharma Ltd (www.neptunepharma.com) in their Joint Venture Partner’s factory in Ningbo, Zhejiang Province. And it has done so successfully: The veterinary medicinal product Trident 500mg/g Powder for Suspension for Fish Treatment (www.trident-50.com), is manufactured onsite under EU…
ECA Foundation releases free GMP WebApp
The ECA Foundation has been providing advanced training and information services in the pharmaceutical industry and especially with regard to pharmaceutical Quality Assurance and GMP compliance for more than 10 years. Now the organisation took advantage of its extensive experience to develop a further free of charge service – the new GMP WebApp.
This new GMP WebApp runs on all smartphones and tablet PCs (Apple and Android platforms) and allows users…
GMP Friction Products Awarded ISO 9001:2008
Internationally Recognized Certification Measures Consistency in Process, Procedure and Quality Performance in Manufacture of Friction Materials
AKRON, OH (March 23, 2011) -- GMP Friction Products, a world leader manufacturing powdered metal friction products for clutch plates and brake pads, recently received certification for ISO 9001:2008.
“ISO 9001:2008 signifies we have taken the extra measure of documenting the policies and standards to ensure consistent compliance with our manufacturing processes,” said Jerry Lynch,…
GMP MANUAL Volume 2 - Validation Procedures by Maas & Peither AG – GMP Publish …
GMP Publishing is launching its new GMP MANUAL Volume 2 – Validation Procedures.
The compendium on validation procedures was written by Dr. Doris Borchert, Dr. Peter Bosshard, Dr. Ralph Gomez, Dr. Michael Hiob, Dr. Christine Oechslein, Max Lazar, Ulrike Reuter, Michael Schulte, Uwe Schwarzat – all international experts and key opinion leaders. They share their detailed understanding of the various aspects of the validation process in clear and comprehensive style…
blue inspection body celebrates 50 GMP audits
Münster (Germany), 20 November 2009. Two years after founding the company and just 18 months after gaining the accreditation blue inspection body GmbH announced today the successful execution of its 50th GMP audit. Further audit trips to China, India, Israel and various European countries have been scheduled already, meaning that in the first quarter 2010 the 75th audit is targeted to be completed. Blue, as a privately organised inspection body,…